Lewis Structure Of Phosgene Ion
Consider the structure of phosgene Cl 2 CO which is shown in the margin. Identify the atoms that correspond to each of the following electron configurations.
4 2 Lewis Structures Problems Chemistry Libretexts
2 draw a line between each pair of connected atoms to represent the electrons in a covalent bond.

Lewis structure of phosgene ion. XeO 2 F 2. Identify the atoms that correspond to each of the following electron configurations. A Lewis structure of OCl- ion is drawn below.
Write the Lewis structures for carbon tetrachloride and phosgene. It is one of the simplest acyl chlorides being formally derived from carbonic acid. Steps for Writing Lewis Structures.
Atom formal charge X 5. The number of valance electrons is Step. The CO distance is 118 Å the CCl distance is 174 Å and the ClCCl angle is 1118.
Between other oxygen atoms there are only single bonds with phosphorous atom. Phosgene is a planar molecule as predicted by VSEPR theory. COCl2 is a chemical compound known by the name phosgene.
However the double bond seems to act much like a nonbonding pair of electrons reducing the Cl C Cl. CI C- Assign a formal charge to each atom in the students Lewis structure. Find valence e- for all atoms.
Steps of drawing the lewis structure of NF3 are explained in detail in this tutorial. Put the least electronegative atom in the center. This step gives you bonding e-.
Write the Lewis structures for carbon tetrachloride and phosgene. Nitrogen trifluoride NF3 lewis structure contains three N-F bonds. NOCl CF 2 Cl 2 HCN.
Identify the atoms that correspond to each of the following electron configurations. Also each oxygen atom has a -1 charge. It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO.
Write the Lewis structures for carbon tetrachloride and phosgene. Write the Lewis structures for carbon tetrachloride and phosgene. 1s 2 2s 2 2p 5 1s 2 2s 2 2p 6 3s 2.
What is the formal charge on each atom. H 2 S NCl 3 OH -. H always goes outside.
Find octet e- for each atom and add them together. A 1s 2 2s 2 2p 5 b 1s 2 2s 2 2p 6 3s 2. Lewis structure of PO 43- ion In the lewis structure of PO 43- three is a double bond between phosphorous atom and one oxygen atom.
Subtract step 1 total from step 2. Then write the Lewis symbol for the common ion formed from each atom. Find number of bonds by diving the number in step 3 by 2 because each bond is.
Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 9892 grammol. The reactivity of the compound is also consistent with an electron deficient boron. What is the formal charge on each atom in CN.
ER 32 32 VE 24 24 SP 4 4 Supporting Materials Periodic Table Fundamental Constants. It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. From the Lewis structure of phosgene we might expect a trigonal planar geometry with 120 bond angles.
Then write the Lewis symbol for the common ion formed from each atom. However the BF bonds are slightly shorter than what is actually expected for BF single bonds indicating that some double bond character is found in the actual molecule. 1s 2 2s 2 2p 5 1s 2 2s 2 2p 6 3s 2.
1 Perry DL et al. There are no remaining electrons hence no need to place them on central atom. Use VSEPR theory to predict the molecular geometry of IF5.
The CH bond is nonpolar since C and H differ by only 035 electronegativity units. Structure and basic properties. Step method to draw lewis structure of Carbonyl Chloride Phosgene Step 1.
Identification of Organic Compound in Industrial Effluent Discharges USEPA-6004-79-016 NTIS-PB-294794 1979 Hazardous Substances Data Bank HSDB. A 1s 2 2s 2 2p 5 b 1s 2 2s 2 2p 6 3s 2. Lewis structure of NF3 can be drawn by using valence electrons of nitrogen and fluorine atoms.
It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. Then write the Lewis symbol for the common ion formed from each atom. Find the total valence electrons for the molecule.
Put two electrons between atoms to form a chemical bond. Assign lone pairs radical electrons and atomic charges where appropriate No Response Calculate the electrons required ER valence electrons VE and shared pairs SP. It is non-flammable in nature and bears a suffocating odor.
Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. C 1 N 0. Add lone pairs so that each atom connected to the central atom gets an octet.
This suggests the best Lewis structure has three BF single bonds and an electron deficient boron. It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. Then write the Lewis symbol for the common ion formed from each atom.
Draw the Lewis structure of phosgene COCl 2. Identify the atoms that correspond to each of the following electron configurations. Use VSEPR theory to predict the molecular geometry of phosgene Cl2CO.
COCl2 Lewis Structure Molecular Geometry Hybridization and Polarity. Cl atom 1 and each O atom -1. A student proposes the following Lewis structure for the phosgene COCI molecule.
In a study of 63 industrial effluents that discharge into surface waters chlorobutane was found in one effluent at a concentration of.

A Student Proposes The Following Lewis Structure For The Phosgene Cocl2 Molecule Assign A Formal Charge To Each Atom In The Student S Lewis Structure Image Src Formal Charge2322492508625533444 J Study Com

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube
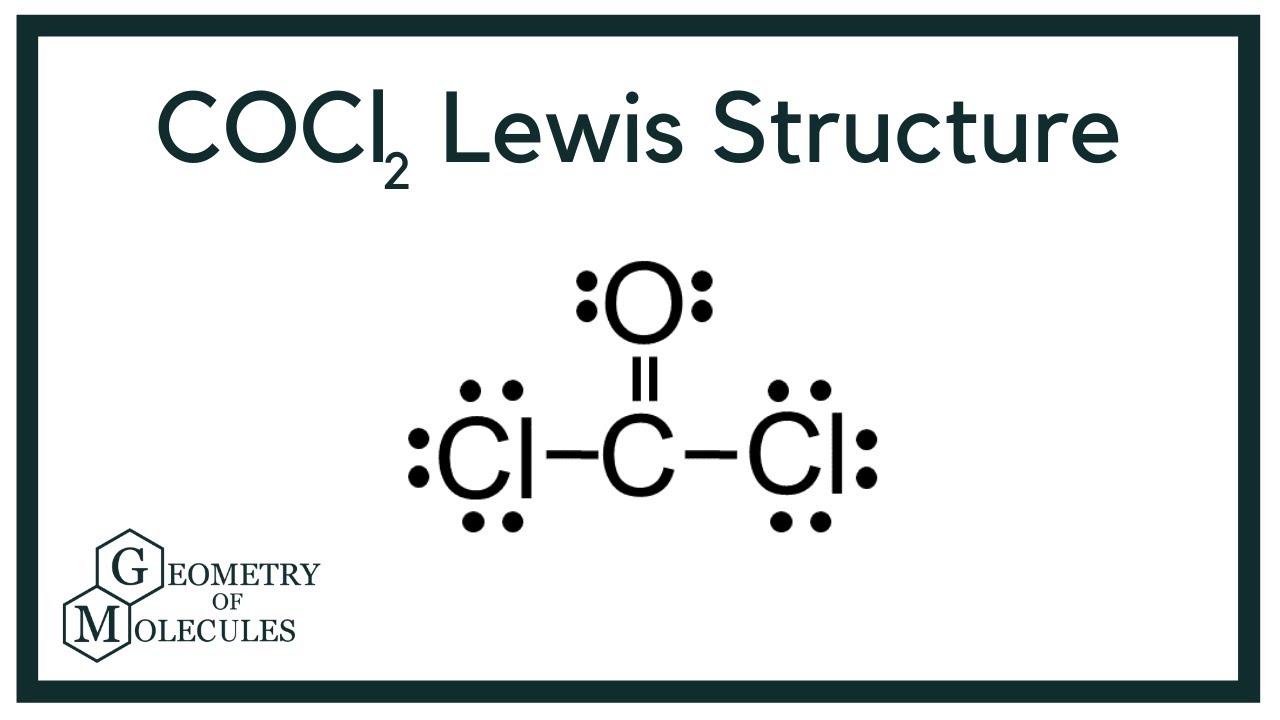
Cocl2 Lewis Structure Phosgene Youtube

Construct The Lewis Structure Model For The Covalent Compound Phosgene Cocl2 Using The Following Steps 1 The Total Number Of Valence Electrons In Cocl2 Is 2 In This Compound Carbon Is

Describe The Hybridization Of The Carbon Atom In The Poisonous Gas Phosgene Cl2co Study Com

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Electronic Structure And Covalent Bonding Ppt Download

Lewis Symbols And Structures Chemistry I

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemi Clutch Prep

Dot And Cross Structure For Cocl2 Phosgene Youtube

Construct The Lewis Structure Model For The Covalent Compound Phosgene Cocl2 Using The Following Steps 1 The Total Number Of Valence Electrons In Cocl2 Is 2 In This Compound Carbon Is
How To Calculate The Formal Charge Of Cocl2
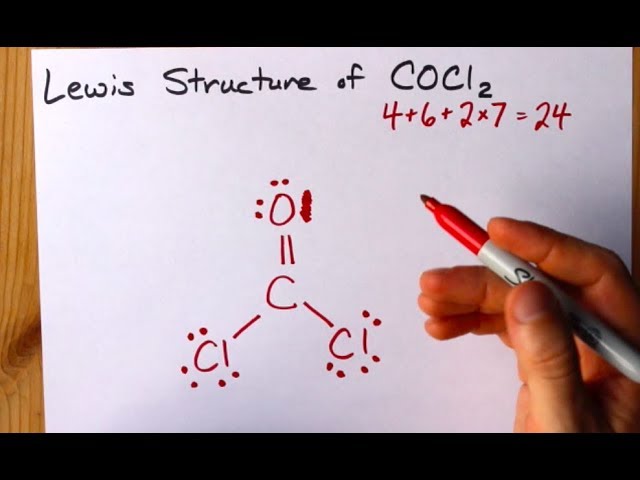
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemi Clutch Prep

6 3 Molecular Shape Introductory Chemistry
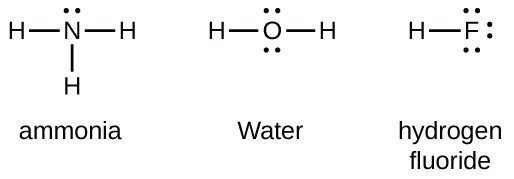
Lewis Symbols And Structures Chemistry For Majors
4 2 Lewis Structures Problems Chemistry Libretexts

Cocl2 Phosgene Molecular Geometry Bond Angles And Electron Geometry Youtube