(nh4)2so4 Grams To Moles
Note that rounding errors may occur so always check the results. There are three definitions equalities of mole.

Molarity Worksheet Teaching Chemistry Chemistry Worksheets Chemistry Classroom
The SI base unit for amount of substance is the mole.

(nh4)2so4 grams to moles. Grams 58443 5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is equal to how many moles. Use this page to learn how to convert between grams FeSO4. Molecular weight of Fe NH42 SO426H2O or mol The SI base unit for amount of substance is the mole.
Note that rounding errors may occur so always check the results. To find the total number of atoms in NH42SO4 Ammonium sulfate well add up the number of each type of atom. 881 x 24 2110 grams.
Mole 602 x 1023 particles. What is the gram formula mass of nh4 2so4. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
Note that rounding errors may occur so always check the results. Molecular weight of NH42 SO4 or grams The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NH42SO3 or 11614012 grams.
We assume you are converting between grams NH42SO3 and mole. 2240 055 moles. 1 grams Fe NH42 SO426H2O is equal to 00025501174584101 mole.
The answer is 29890402e23 grams. Ammonium sulfate - YouTube. 157 21 moles.
Use this page to learn how to convert between moles NH4. Moles 2000 169873 1177 moles. This compound is also known as Ammonium Sulfate.
Molecular weight of NH42SO3 or mol The SI base unit for amount of substance is the mole. How many moles are in 325mL of 125M NH42SO4. Note that rounding errors may occur so always check the results.
The SI base unit for amount of substance is the mole. The formula mass of NH4 2 SO4 is 132 atomic mass units. 1 grams NH42SO3 is equal to 00086102890198495 mole.
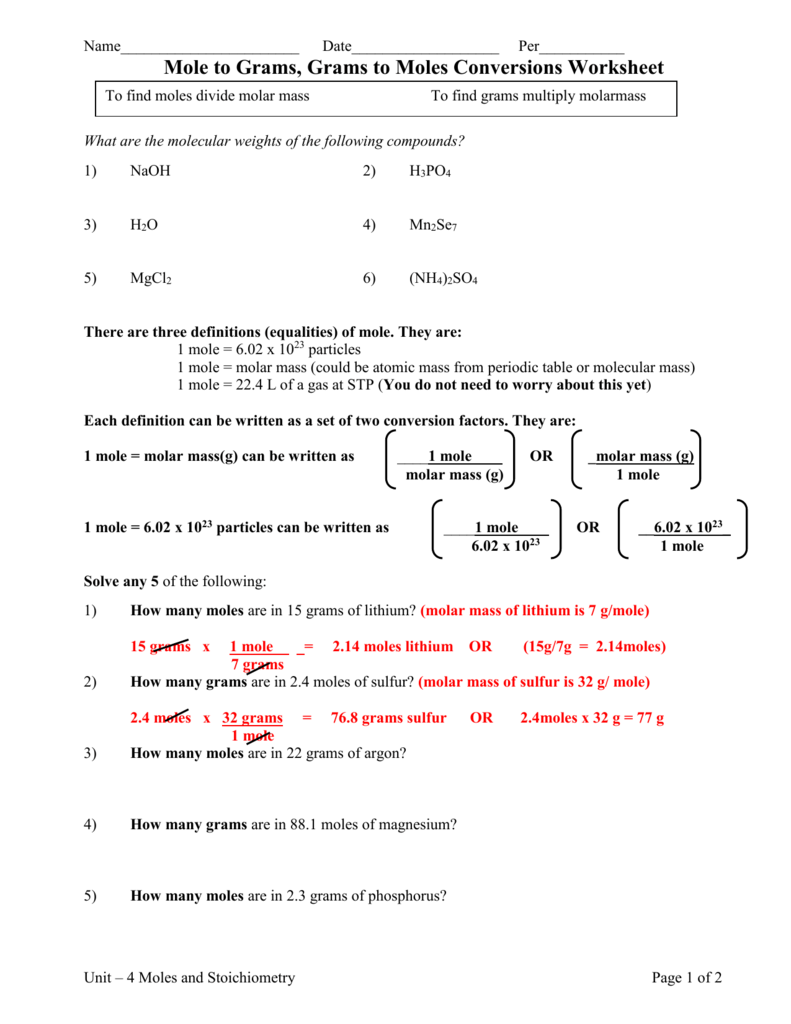
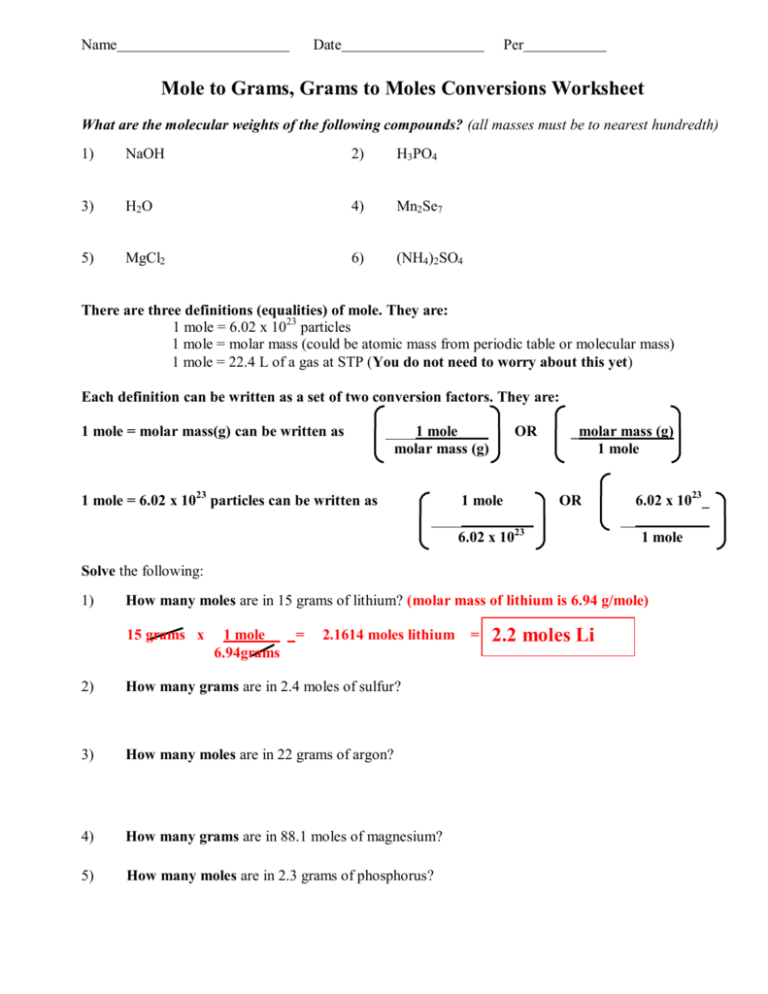
5 How many moles are in 23 grams of phosphorus. Grams mole molar mass. Molar Mass Molecular Weight of NH42SO4.
Note that rounding errors may occur so always check the results. 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 L of a gas at STP You do not need to worry about this yet. Use this page to learn how to convert between moles NH42SO4 and gram.
Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. The SI base unit for amount of substance is the mole. 2 How many grams are in 24 moles of sulfur.
We assume you are converting between moles NH42CrO4 and gram. Mote1985 20 10 months ago. 1 grams NH42SO4 is equal to 00075677586841544 mole.
1 mole is equal to 1 moles NH42CrO4 or 15207062 grams. The following formula can be used to calculate the moles present in eqrm 366 times 10-2g eq of eqrm NH_4_2SO_4 eq. Molecular weight of NH42SO4 or mol This compound is also known as Ammonium Sulfate.
Hello In this case since the molarity is defined as the ratio of the moles of the solute to the volume of the solution in liters. You can view more details on each measurement unit. The answer is 11614012.
To find the mass of 002 moles of beryllium iodide simply multiply the number of moles by the molar mass in conversion factor form. 3 How many moles are in 22 grams of argon. 1 mole is equal to 1 moles NH42 SO4 or 13213952 grams.
Eqrm ndfracmM eq Where n moles m mass. Molecular weight of FeSO4. 24 x 32 77 grams.
4 How many grams are in 881 moles of magnesium. 1 mole is equal to 1 moles NH42SO4 or 13213952 grams. NH42SO46H2O is equal to 00025501174584101 mole.
Well use the Mole Map as our guide to setting up and understanding how to convert between moles grams liters and particles molecules atoms. For the given volume and molarity we solve for the moles of ammonium sulfate solute as shown below. Molecular weight of NH42CrO4 or grams The SI base unit for amount of substance is the mole.
Solve any 15 of the following. The SI base unit for amount of substance is the mole. 1 How many moles are in 15 grams of lithium.
Note that rounding errors may occur so always check the results. The molar mass of NH4 is determined by multiplying 1 molecule 1 mol NH4 6022e23 Avogadros constant 18 g mol NH4. The small number after the.
How many grams NH42SO3 in 1 mol. You can view more details on each measurement unit. Molar Mass Molecular Weight of NH42SO4.
The small number after the element symbol is. Use this page to learn how to convert between moles NH42SO3 and gram.

1 What Is The Molar Mass Of Ammonium Sulfate Chegg Com

Mathematical Relationships With Chemical Formulas Ppt Download

How Many Grams Of Oxygen Are In 4 7780 Grams Of Chegg Com

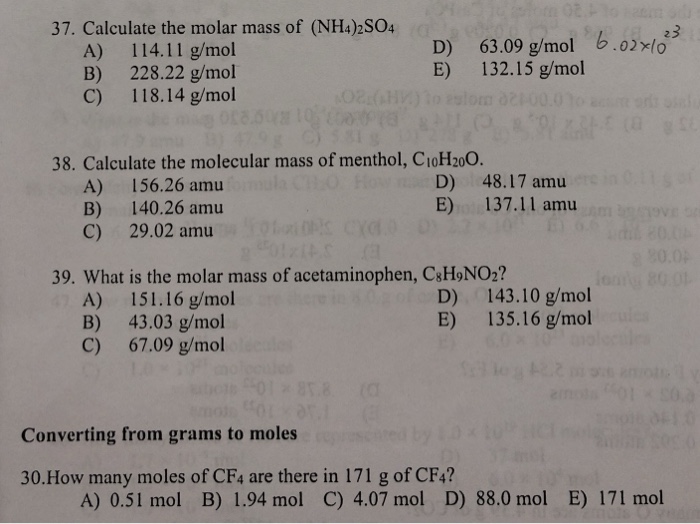
Calculate The Molar Mass Of Nh4 2so4 Ammonium Sulfate Molar Mass Practice Youtube
Calculate The Amount Of Nh4 2so4 In G Which Must Be Added To 500 Ml Of 0 2 M Nh3 To Sarthaks Econnect Largest Online Education Community

How Many Moles Are In 123 0g Nh4 2so4 Brainly Com

Introductory Material Ppt Download

How To Find The Number Of Atoms In Nh4 2so4 Ammonium Sulfate Youtube
Calculate The Amount Of Nh4 2so4 In G Which Must Be Added To 500 Ml Of 0 2 M Nh3 To Sarthaks Econnect Largest Online Education Community
37 Calculate The Molar Mass Of Nh4 2so4 A 114 11 Chegg Com

How To Find The Number Of Atoms In Nh4 2so4 Ammonium Sulfate Youtube
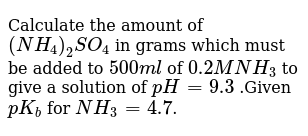
Calculate The Amount Of Nh4 2so4 In Grams Which Must Ve Added To
Molar Mass Molecular Weight Of Nh4 2so4 Ammonium Sulfate Youtube
Grams To Moles Moles To Grams Practice
Molar Mass Molecular Weight Of Nh4 2so4 Ammonium Sulfate Youtube

5 How Many Grams Of Nh4 2so4 Molar Mass 98 1 Chegg Com

Question 3 Given The Reaction Chegg Com
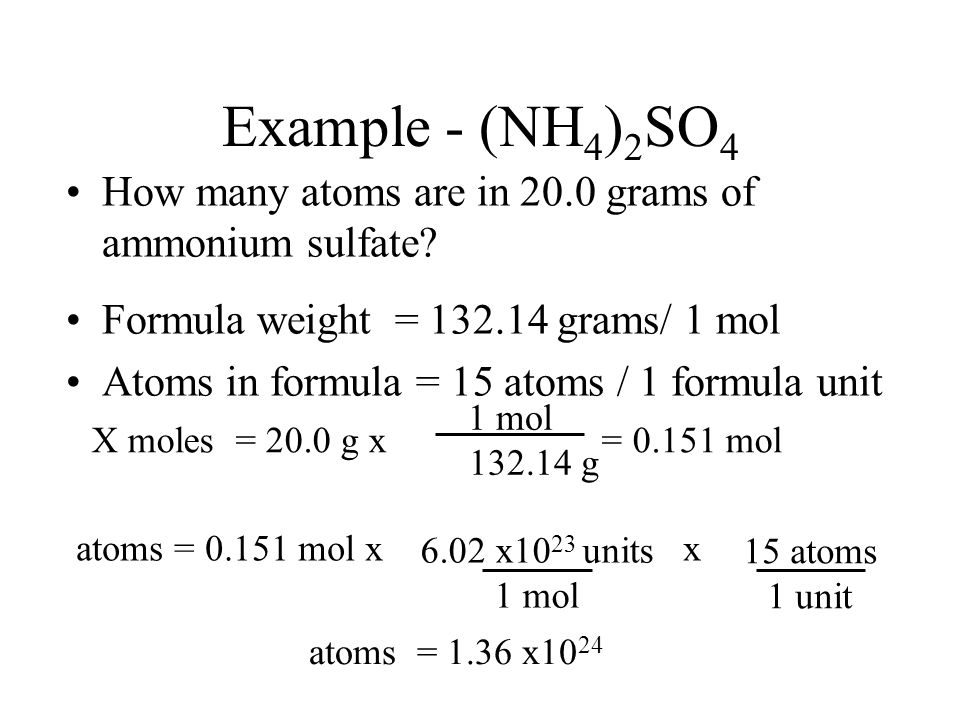
Introductory Material Ppt Download