The Lewis Structure Of C2h2 Has 1 Triple Bond
O 2 double bonds and 2 lone pairs. There are different type of compounds or ions which can be represented by Lewis dot structures.

Draw The Lewis Dot Structure Of C2h4 C2h2 And Co2 Brainly In
C has four valence electrons two in 2s and two in 2p and H has one one in 1s.

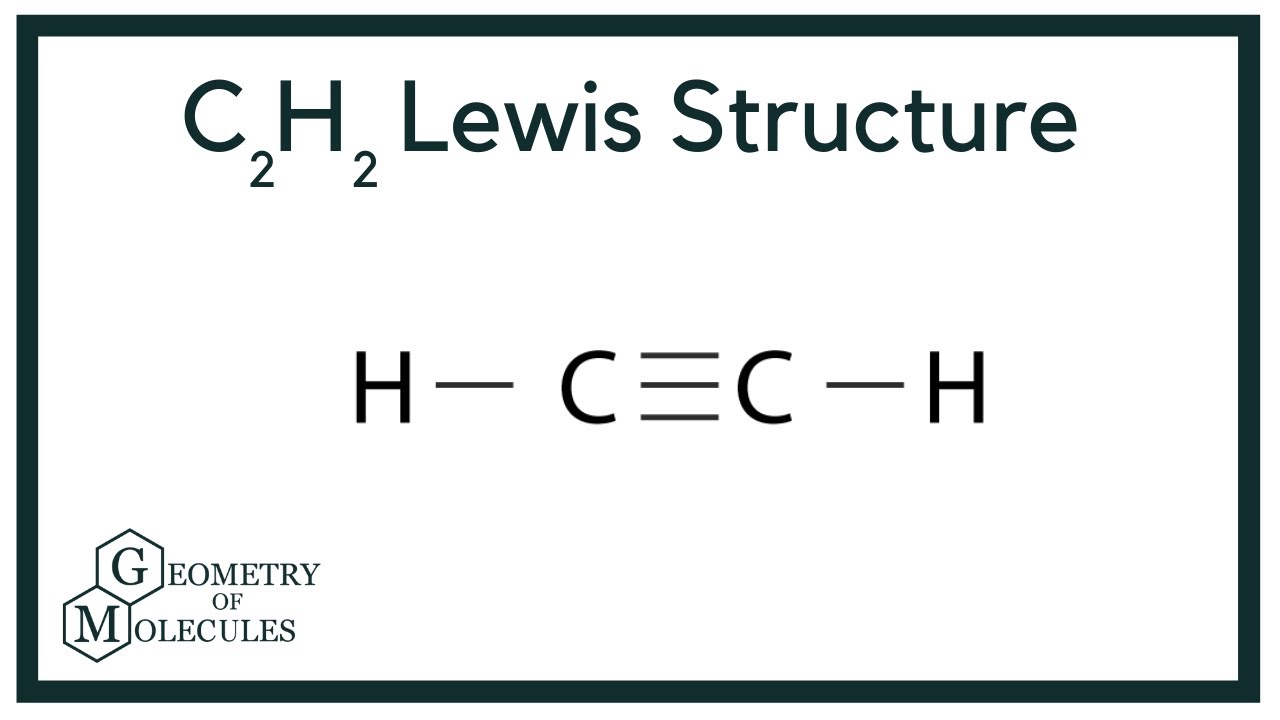
The lewis structure of c2h2 has 1 triple bond. For the HCCH Lewis structure youll need to form a triple bond between the two carbon atoms. Triple bonds are always shorter than single bonds. There are no lone pairs on carbon or hydrogen atoms.
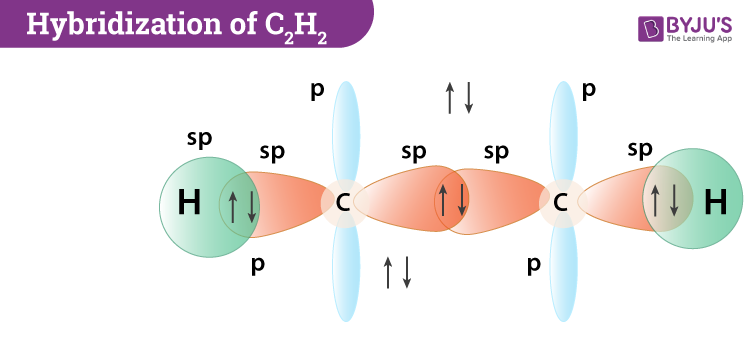
For each C one can explain the bonds through sp hybridization a triple bond and one single bond. Is C2H2 trigonal planar. So now youre redy to answer the question - each acetylene molecule has one triple bond.
This means that the two carbo natoms must be bonded to each other. This will account for 4 of the 10 valence electrons 2 for each hydrogen - carbon single bond. Single bond s and triple bond s present 1.
Ethyne C2H2 has a triple bond between the two carbon atoms. Since there is only one possible lewis structure C2H2 does not have resonance. In this tutorial we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. Find more Chemistry widgets in WolframAlpha. Ethane C2H6 ethene C2H4 and ethyne C2H2 each consist of two carbon atoms but a different number of hydrogen atoms.
Arrange electrons until both carbon and nitrogen get a triple bond giving an octet and hydrogen has 2. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. Commonly called acetylene has o 2 single bonds 1 triple bond and 1 lone pair.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. In order to calculate the formal charges for C2H2 well use the equationFormal charge of valence electrons nonbonding val electrons bonding ele. Hydrogen has one bond and no lone pairs.
The number of sigma and pi-bonds are 6 and 2 respectively. 4 pairs of valence electrons The correct lewis structure for a molecule of the compound c2h2 contains 1 triple bond If two covalently bonded atoms move farther than the distance of bond length. Question 7 1 pts The best Lewis structure for ethyne C2H2.
Click to see full answer. In order for them to have completel octets you need to form a triple bond which will use up the remaining 6 valence electrons between them. The C2H2 molecule contains a triple bond between the two carbon atoms one of which is a sigma bond and two of which are pi bonds.
A molecule has resonance if more than one lewis structure. Why is C2H2 a triple bond. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
When figuring out whether to place a double or triple bond you should always look at the number of valence electrons present as well as the number of bonds a central atom is likely to form. Acetylene Ethyne Lewis Structure. As we know that carbon has 4 valence electrons and hydrogen has 1 valence electron.
There is a total of ten valence electrons. It has 3 σ-bond and 2 π bond. Solution for Which molecule has a triple bond.
O 2 single bonds 1 double bond and 2 lone pairs. In the case of C2H2 you are right there is only one possible lewis structure. Match each description with the appropriate molecule.
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2. C 2 H 2. According to Lewis-dot structure there are 16 number of bonding electrons and 0 number of non-bonding electrons.
Another good way to know whether to use double or single bonds is to calculate the formal charge on each atom in the. Drawing the Lewis Structure for C2H2 Ethyne or Acetylene For C2H2 you have a total of 10 valence electrons to work with. Put carbon in the center and arrange hydrogen and nitrogen atoms on the sides.
How many types of law are there in physics. O 2 single bonds 1 triple bond and no lone pairs. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
The Lewis structure will thus look like this. From the Lewis structure one can see that there is a triple bond between the two Cs. Ethane C2H6 total number of valence electrons in the molecule.
Also question is how do you know if a Lewis structure has a double bond. So ive used all my valence electrons Hydrogens are fine. Bonding electron groups associated with each C atom.
C2H2 will have linear trigonal planar tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped octahedral square pyramidal square planar electronic geometry and linear trigonal planar tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped octahedral square.
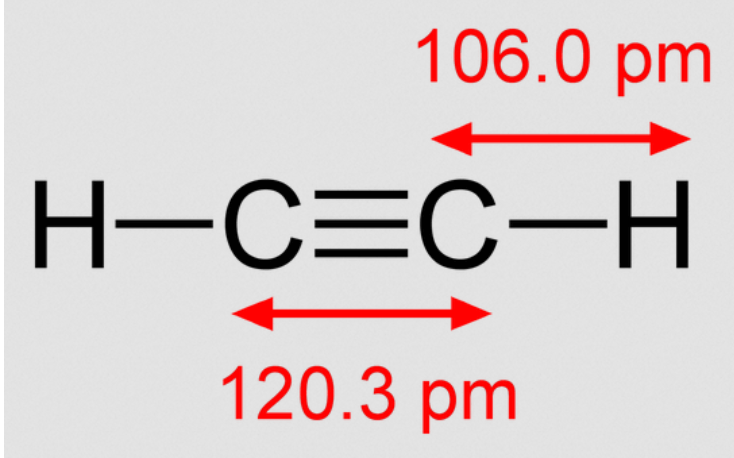
Ethyne Acetylene Structure Properties And Uses Of C2h2

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube

Draw The Lewis Structure For Acetylene C2 Clutch Prep
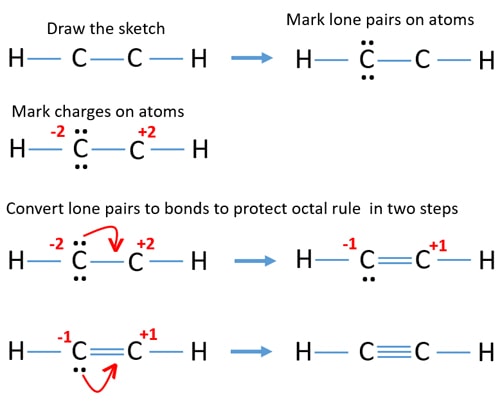
C2h2 Acetylene Ethyne Lewis Structure

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Lewis Structure For C2h2 Ethyne

Is C2h2 Polar Or Nonpolar All About C2h2 Polarity
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

What Is The Number Of Sigma And Pi Bond In C2h2 Quora

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube
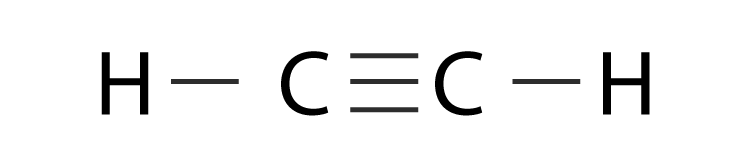
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
C2h2 Lewis Structure Ethyne Or Acetylene Youtube

Molecular Geometry Of Acetylene Chemistry Stack Exchange

C2h2 Acetylene Ethyne Lewis Structure
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C3h4 Lewis Structure How To Draw The Lewis Structure For Ch3cch Youtube
What Is The Structure Of C2h2 On The Basis Of Hybridization Quora