What Is The Electron Geometry Of H2o
Enter the electron geometry of the molecule. This structure gives a water molecule a bent molecular shape.

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula
The resulting shape is bent with an H-O-H angle of 1045.
What is the electron geometry of h2o. The electron pair geometry of water with the chemical formula H2O is a tetrahedral. Nonlinear with no lone pairs on the Cl atom. What is the electronic of ClF3.
Enter the electronic geometry of the molecule. Part B What is the electron geometry of H2O. Because the water molecule has four electron domains the two hydrogen atoms and the two lone.
In H 2 O molecule the Oxygen atom forms two single sigma bonds with Hydrogen atoms. The hydrogen atoms are as far apart as possible at 120o. Enter the electronic geometry of the molecule.
Correct answer to the question. Enter the electronic geometry of the molecule. Part B What is the electron geometry of H2O.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. Determine the electron geometry eg and molecular geometry mg of PF5. Correct answer - What is the electron geometry and molecular geometry of.
What is the electronic geometry of BBr3. As the bonding of the oxygen will form an angle close to 120 this molecule present V-Shape or Bent. How to Determine Electron Geometry.
What is the electron geometry of h2o Molecular geometry also known as the molecular structure is the three-dimensional structure or arrangement of atoms in a molecule. To determine the shapes of molecules we must become. Enter the electronic geometry of the molecule.
Linear with lone pairs on the Cl atom. How many lone pairs of electrons are on the S atom in SF 4. Which of the following best describes ClF 2-.
Correct answer to the question What is the electronic geometry of h2o. The electron geometry should be trigonal planar as it can be noted in the attached image. H2O Molecular Geometry.
This structure gives a water molecule a bent molecular shape. Understanding the molecular structure of a compound can help determine the polarity reactivity phase of matter color magnetism as well as the biological activity. Homework solution attached Purchase this answer to view it This homework is solved by this writer.
In this video we look at the electron geometry for Water H2O. Enter the electronic geometry of the molecule. This molecule is electron deficient and does not follow the octet rule because it has only 6 valence electrons.
Because the water molecule has four electron domains the two hydrogen atoms and the two lone. The molecular geometry of any molecule depends on its Lewis structure the arrangement of atoms and its electrons. Enter the electron geometry of the molecule.
Nonlinear with lone. Although these two Hydrogen atoms are arranged symmetrically in the plane the two lone pairs of electrons on the Oxygen atom push these atoms. This means there are four electron pairs arranged in a tetrahedral shape.
LiBr Na NaO H2O Ne. It comprises two or several types of atoms. What is the electronic geometry of h2o.
What is the electronic geometry of H2O. You can always ask and chat with this. The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms.
This molecule presents two oxygen atoms bonded to the S atom and one electronic couple. Answered Jan 10 2019. Asked Jan 10 2019 in Chemistry by Beth401.
Linear with no lone pairs on the Cl atom. There are two bonding pairs and two lone pairs. A molecule is the smallest fundamental unit of a pure chemical compound.
With 4 electron regions the VSEPR electronic geometry is. What is the electronic geometry of h2o. What is the predicted electron-pair geometry of a molecule of H2O.
Answered Jan 10 2019 by airjatt23. The geometry of electron pairs in water bonding and non-bonding is tetrahedral to a first approximation. It has a molecular geometry that is.
And molecular geometry is BH3. Water or H2O has 8 electrons around the central oxygen atom. Moreover these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule.
This is trigonal planar geometry. What is the electron geometry and molecular geometry of H2O.
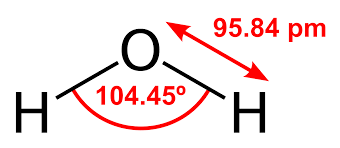
H2o Molecular Geometry Lewis Structure Shape And Bond Angles

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules
What Is The Shape And Geometry Of H2o And Xef4 Quora

Effect Of Loan Electron Pairs In Shaping H2o Molecule

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Digital Kemistry Best Chemistry Animated Blogs Why Are Bond Angles Of H2o Nh3 104 5 And 107 5 Chemistry Basic Physics Chemistry Study Guide

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula
H2o Molecular Geometry Lewis Structure Shape And Bond Angles
What Is The Shape And Geometry Of H2o And Xef4 Quora

Ch3cl Lewis Structure Chloromethane In 2021 Lewis Molecules Methylation



