Xef2 Lewis Structure Polar Or Nonpolar
A Xef4 Lewis Structure Polar Or Nonpolar Hình ảnh. In addition to diatomic molecules with identical atoms the most common type of non-polar compounds are the hydrocarbons.

Xef2 Lewis Structure Xenon Difluoride In 2021 Lewis Molecules Math Equations
Hence Xenon Difluoride is nonpolar as there is no polarity observed in the molecule.

Xef2 lewis structure polar or nonpolar. Hope that helps. How it is polar or nonpolar. For the rest we need to consider the molecular geometry.
The electron geometry is octahedral and the hybridization is sp3d2. Describe the orbitals that overlap to form all bonds in SO3. HCN Lewis structure Molecular structure and shape Most electronegative Contains a polar polar or nonpolar.
According to Vallence bond theory what is the hybridization of all atoms in SO 3. Similarly four dipoles in a planar structure of. Xef5 polar or nonpolar.
HCN Lewis structure Molecular structure and shape Most electronegative Contains a polar polar or nonpolar. Detailed Explanation As we have already known there are certain conditions for a molecule to be polar or nonpolar in the above definitions of the polar molecule and nonpolar. The molecule has octahedral electron geometry and square.
What is a non polar solute. XeF2 is nonpolar in nature because of its linear-shaped geometry having fluorine atoms symmetrically on both sides of the xenon atom. In XeF2 molecule two fluorine atoms are arranged symmetrically on the outside with the central atom Xenon in the middle.
An example is solid iodine I2 dissolved in liquid bromine Br2. Hãy xem xef4 lewis structure polar or nonpolar hình ảnh- bạn cũng có thể quan tâm is xef4 polar or nonpolar or how do you know if a lewis structure is polar or nonpolar. If it is not completely symmetrical AND it has polar bonds it will be polar.
Symmetrical the dipoles of both Xe-F bonds get canceled by each other resulting in a nonpolar XeF2 molecule. Polarity results from the net dipole moment in the molecule. In your example of SF_4 the Lewis Structure would look like this.
Why is XeF2 a nonpolar molecule. However Xe-F bond is polar because the electronegativity of Xe and F is different but the polarity of both Xe-F bonds gets canceled by each other resulting in a nonpolar XeF2 molecule. The polarity is best found by first drawing the Lewis dot structure for BrF5.
December 11 2020 Posted by Uncategorized No Comments. Strongest atom bond YN. It is used in the semiconductor industry rocket propellant and military applications.
Thus the molecule is polar. Strongest atom bond YN. You can see that there is a lone.
Next draw the 3-dimensional structure. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. Xe has one F on the left and another on the right.
Since they are the same atoms they have the same electronegativity electrons pulling power. This explains why XeF2 has a linear molecular shape. Solvents possess molecules with similar electronegativity valuesThe easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and if necessary check its molecular geometry.
We can immediately identify some molecules as nonpolarAny molecule that has zero lone pairs nonbonding pairs of electrons on the central atom and for which all the outer atoms are the same is nonpolarSo we can elimiate CCl4 and PBr5 as being polar. XeF2 is a nonpolar molecule despite two Xe-F bonds are polar. Even though C-O Xe-F bond is polar due to differences in electronegativity but the net dipole moment of CO2 XeF2 XeF4 is zero due to their symmetrical structures.
Xef2 Polar Or Nonpolar. Is XeF4 polar or nonpolar. There is no net dipole moment in the compound due to the arrangement of the valence electrons in symmetry.
NON-POLAR SOLUTE NON-POLAR SOLVENT. XeF2 Lewis structure Molecular structure and shape Most electronegative Contains a polar polar or nonpolar. In this article we will discuss ClF3 lewis dot structure polar or non-polar its molecular geometry or shape bond angle hybridization etc.
The XeF2 has a linear molecular geometry and Xe-F bonds are symmetrical to each other as a result the net dipole moment becomes zero. Having one on the left and another on the right makes the molecule symmetric thus cancelling the dipole moment making XeF2 non-polar. Add formal charges and bond dipoles to all lewis dot structures.
But due to the linear structure ie. PCIs Lewis structure Molecular. XeF2 Molecular structure and shape Lewis structure Most electronegative Contains a polar polar or nonpolar.
Nonpolar compounds either have no polar bonds or contain symmetrical polar bonds. Learn to determine if XeF2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look an. Are SO3XeF2IF2- polar or nonpolar.

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular
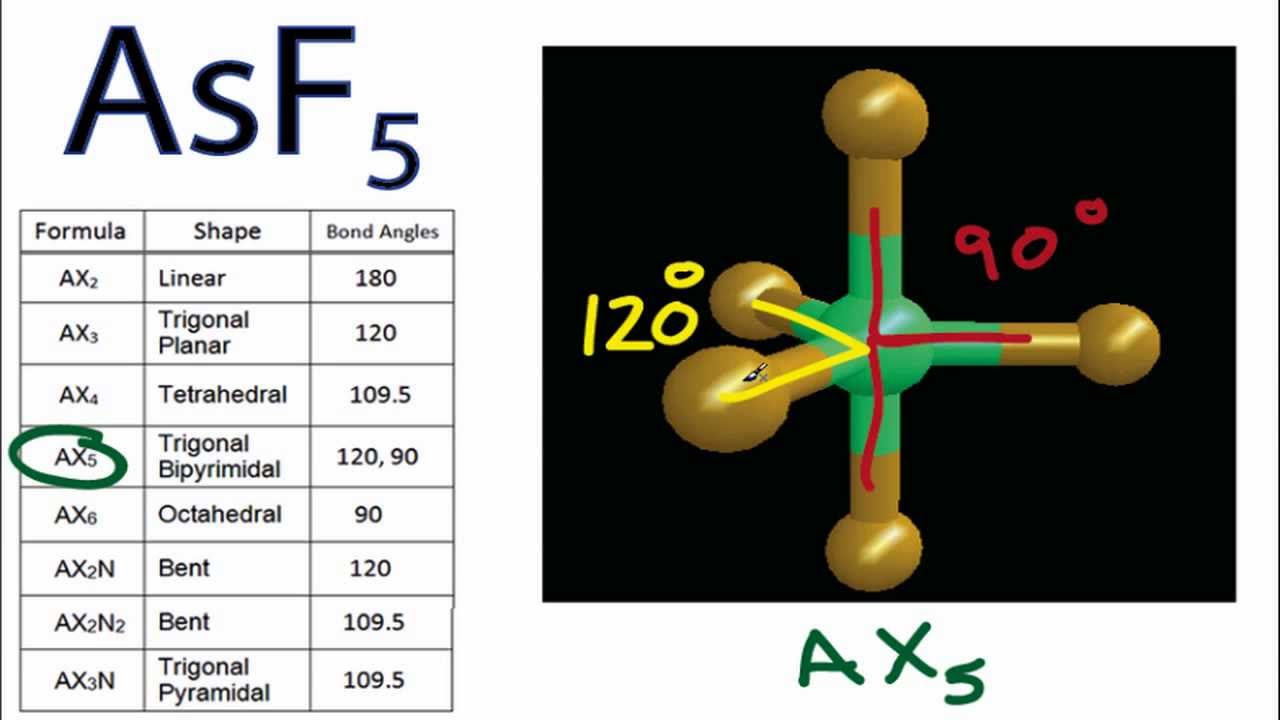
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

