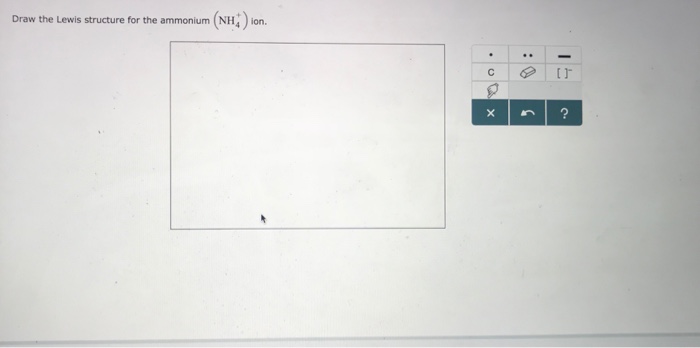
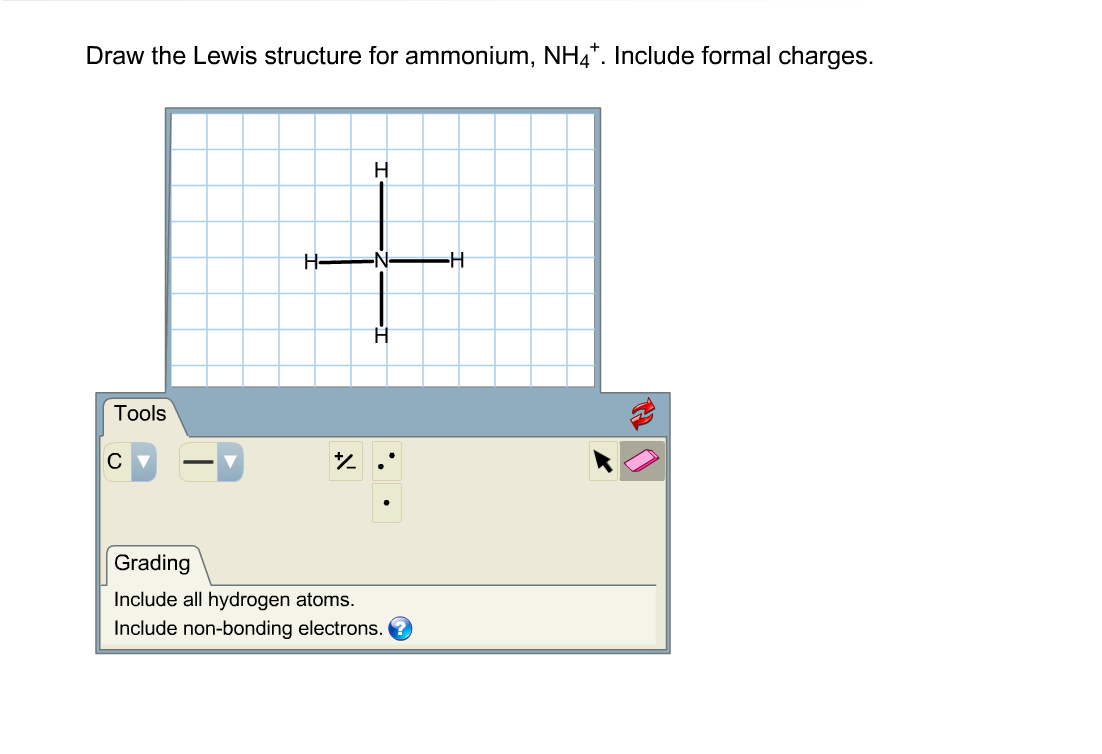
Draw The Lewis Structure For Ammonium, Nh4 . Include Formal Charges.
This problem has been solved. Draw the Lewis Dot Structure for the Hydrogen atom.
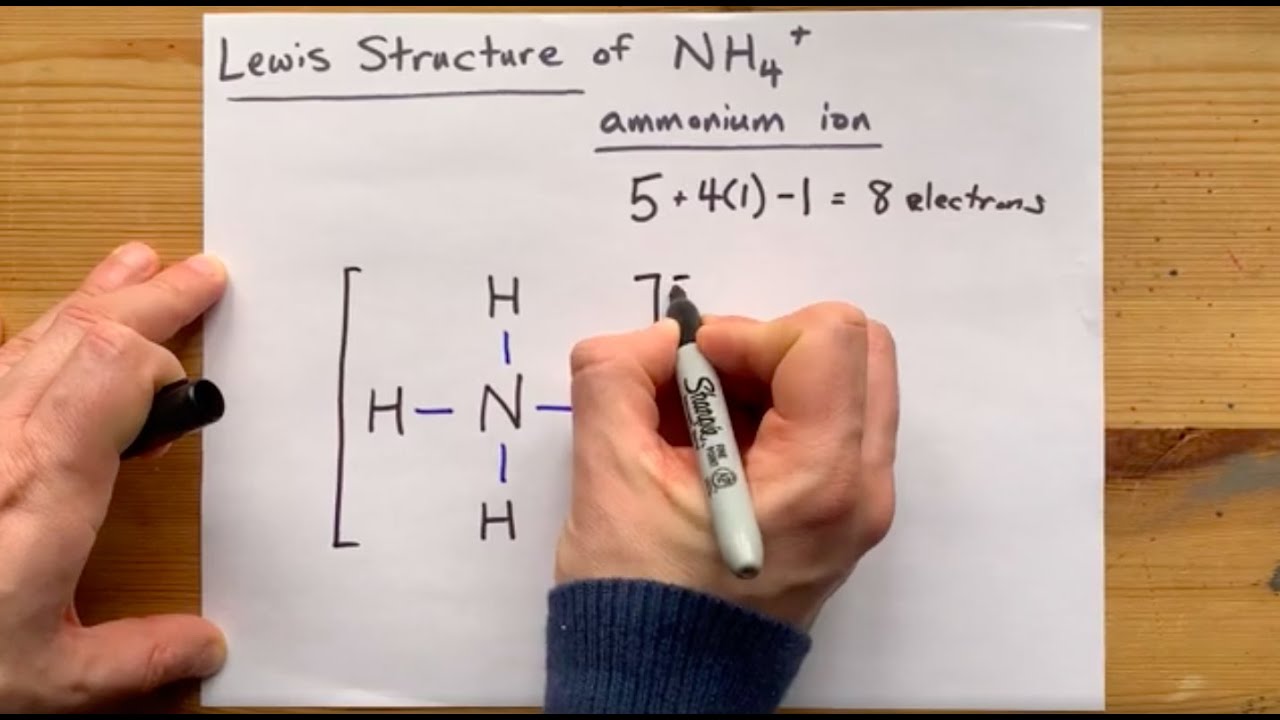
Lewis Structure Of Nh4 Ammonium Ion Youtube
The definitive way to determine formal charge is to draw the Lewis structure.

Draw the lewis structure for ammonium, nh4 . include formal charges.. Draw The Lewis Dot Structure For Nh4 NH4 Lewis Structure YouTube GRE CHEMISTRY Lewis Structure Tang 05 lewis dot diagrams. Draw the Lewis structure for the ammonium ion NH4. Its one of the few positive polyatomic ions youll work with.
For example consider the ammonium ion NH 4 which contains 9 5 from N and 1 from each of the four H atoms 1 8 electrons. These kinds of structures can also be shown by representing each of the bonds with two dots. The bonding electrons are shared equally between the bonded atoms.
The lone pair electrons belong entirely to the central atom. 605a Determine the formal charg on atoms in NH4 224. The formal charge on each hydrogen atom is therefore formal.
Steps of drawing the lewis structure of NH 4 are explained in this tutorial. 605 Resonance Formal Charge 1627. The Lewis Dot Structure for NH4.
Ammonium formula nh4 lewis structure dot draw write carbonate chemical ion. One electron is subtracted because the entire molecule has a 1 charge. 605b Determine the formal charge on atoms in H2CO 317.
A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc. Were being asked to draw a Lewis structure for NH4. There is a 1 charge on nitrogen atom.
NH 4 Ammonium ion Lewis Structure. When we write the Lewis structure for NH 4 we need to subtract one valence electron from out total. The formal charge on N is zero in NH_3 and 1 in NH_4.
Nh4 lewis structure draw dot bonds atoms lone placing connecting grid pairs include electrons answers transcribed them. Chargeleft N right 5-left 0dfrac82 right 0 Each hydrogen atom in has one bonding pair. One can draw the 3-dimensional structure of an atom once they have the Lewis Structure of an atom.
Be and B dont need 8 valence electrons. When the Lewis structure of an ion is written the entire structure is placed in brackets and the charge is written as a superscript on the upper right outside of the brackets. 606a Draw the Lewis structure for I3- 116.
Nitrogen having 5 valence shell electrons along with 4. SO 4 2- N 2 O XeO 3. Show the calculation of the formal charge on nitrogen and hydrogen in the ammonium ion.
Chargeleft H right 1-left 0dfrac22 right 0. H only needs 2 valence electrons. Check the Formal Charges to make sure you have the best Lewis Structure.
N 5A 1 5 e 5 e. Because NH 4 is a positive ion called a cation that means it lost one electron. Heptane is an unbranched alkane with the condensed structure CHCHCHCH CHCHCHDraw the comple.
See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. S and P sometimes have more than 8 val. 606 Exceptions to the Octet Rule 848.
The Lewis structure is from wwwchemteaminfo. Using Equation ref851 the formal charge on the nitrogen atom is therefore formal. All the H is connected to the carbon written.
Solution for Draw the Lewis structure for ammonium NH4NH4. The Lewis Dot Structure for NH4 Ammonium is shown above. H 1A 4 1 e 4 e.
Since Hydrogen is in Group I it has one 1 valence electron in its shell. 606b Draw the Lewis structure for ClF4 141. Draw the best Lewis structure include formal charges for the following molecule.
In NH 4 ammonium ion lewis structure there are four sigma bonds around nitrogen atom. Show the calculation of the formal charge on nitrogen and hydrogen in the ammonium ion. Draw The Lewis Structure For Ammonium NH4 Include Formal Charges.
Notable Exceptions to the Octet Rule. NH 4 is one of the polyatomic ions that you should memorize. This type of Lewis dot structure is represented by an atomic symbol and a series of dots.
The 3-dimensional geometrical structure of ammonium NH4 is referred to as Tetrahedral. Since hydrogen cannot be a central atom N is the central atoms. Draw the Lewis structure for the ammonium ion NH4.
Each atom in the bond has a full valence shell with nitrogen having access to eight electrons and each hydrogen having access to two this is why. Specific rules decide the arrangement of electrons in a molecule as per. Draw The Lewis Structure For Ammonium NH4 Include Formal Charges.
Drawing the Lewis Structure for NH 4 Viewing Notes.
What Is The Nh4 Lewis Structure Quora

How To Calculate The Formal Charges For Nh4 Ammonium Ion Youtube

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
What Is The Nh4 Lewis Structure Quora

What Is The Nh4 Lewis Structure Quora

Vsepr For 4 Electron Clouds Video Khan Academy
Ammonium Carbonate Nh4 2co3 Pubchem
How Do I Draw This Structure Without Brackets I Want Chegg Com
How To Draw Lewis Structures On Sapling Drawing Easy
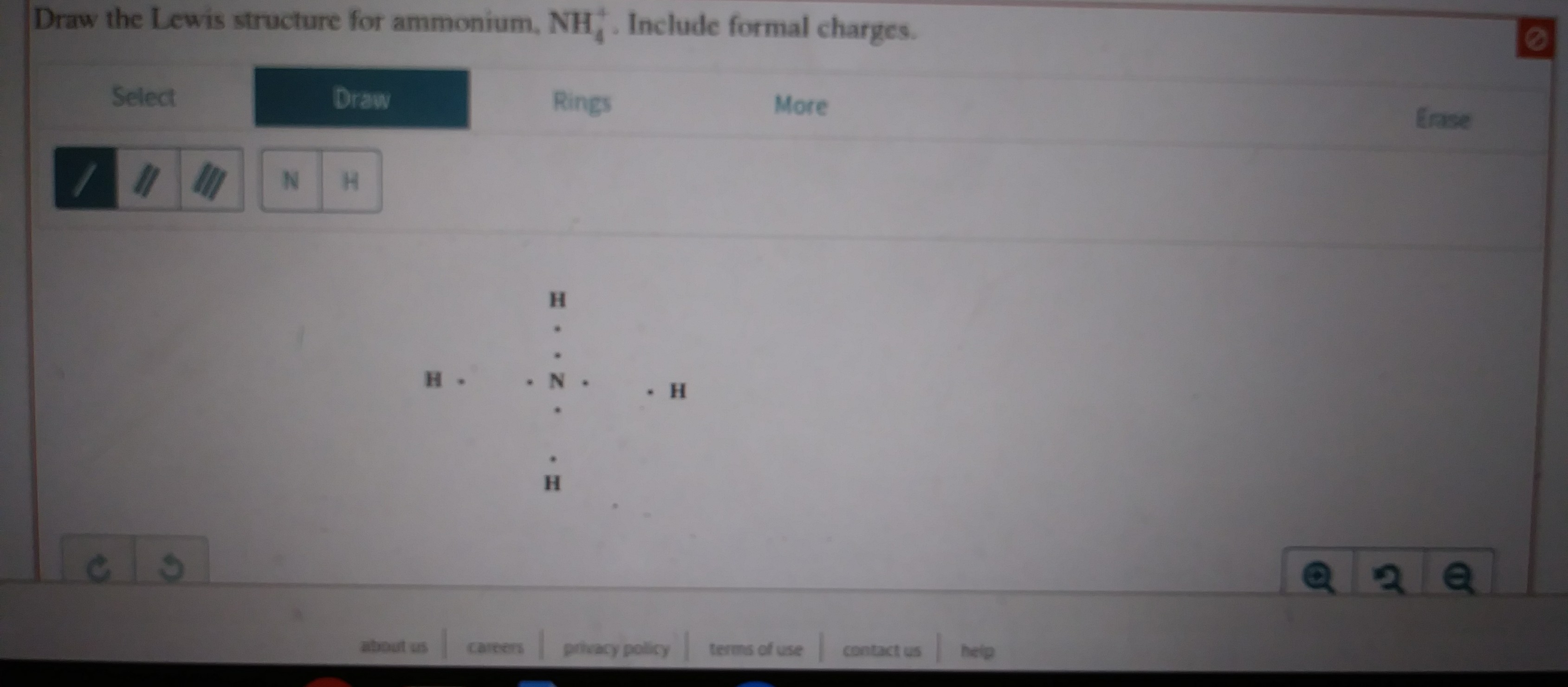
Answered Draw The Lewis Structure For Ammonium Bartleby

Formal Charge Problems 1 Nh4 Youtube

How To Draw The Nh4 2so4 Lewis Dot Structure Ammonium Sulfate Youtube
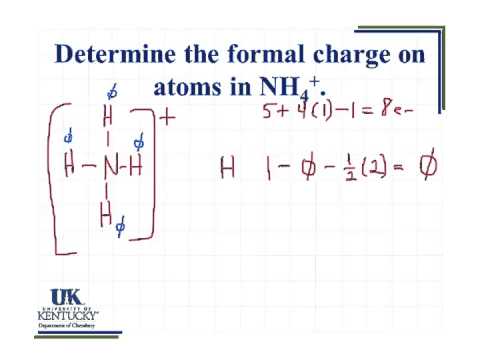
6 05 Determine The Formal Charge On Atoms In Nh4 Youtube
Draw The Lewis Structure For The Ammonium Nh4 Ion Chegg Com
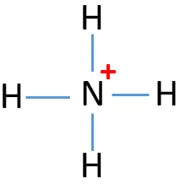
Nh4 Ammonium Ion Lewis Structure

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots

Chemistry 101 Drawing Lewis Structures Polyatomic Ions Ammonium Youtube