Pbr3 Lewis Structure Electron Geometry
Drawing PBr3 Lewis Structure is very easy to by using the following method. Draw the Lewis dot structure for PBr3.
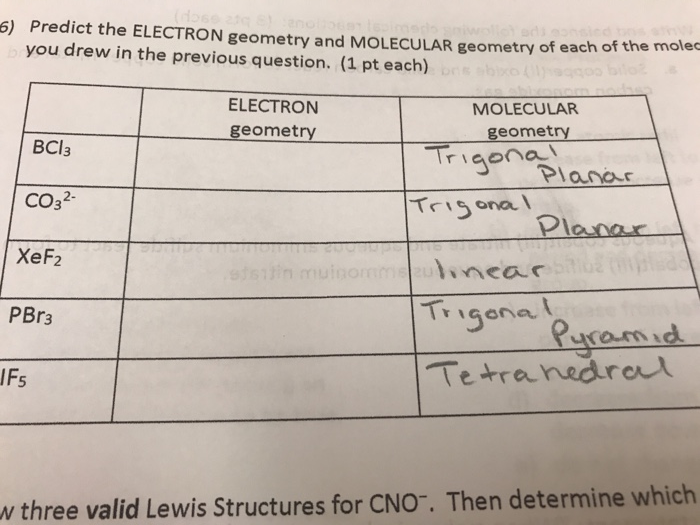
Predict The Electron Geometry And Molecular Geometry Chegg Com
PCl3 Hybridization The hybridization of PCl3 can be determined once we know the Lewis dot structure of this molecule.
Pbr3 lewis structure electron geometry. Tribromide Br3- CID 77881 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Determine the electron geometry and molecular shape of this molecule. PBr3 has one P atom with an electronegativity value of 219 and three Br atoms each with a 296 value of electronegativity.
Here in this compound there is one molecule of Phosphorus having five valence electrons and five molecules of Bromine having seven valence electrons each. 5 for P 7 for each bromine atom respectively. Tetrahedral trigonal pyramidal trigonal planar bent Click Save and Submit to save and submit.
A step-by-step explanation of how to draw the IBr3 Lewis Dot StructureFor the IBr3 structure use the periodic table to find the total number of valence elec. The phosphorus tribromide chemical formula is PBr3. When you look at the Lewis Structure of the molecule you can see that electrons arrangement is in a tetrahedral geometry.
What is the molecular geometry of PBr 3. What is the electron geometry and shape of the molecule. Is the molecule polar or non polar.
This leads to four electron pairs and thus Br in hydrogen bromide is tetrahedral with a dihedral angle of 1090 to give sp3 hybridization. The electron geometry for the. Here in this post we.
Hence the electron geometry of Phosphorus Trichloride is tetrahedral. With such a high difference we have polar bonds between P and Br where there is a partial charge near P and a partial charge near Br in each of the bonds. Draw the Lewis Structure for phosphorus tribromide Pbr3.
Give reason for your answer. A step-by-step explanation of how to draw the PBr3 Lewis Dot Structure Phosphorus TribromideFor the PBr3 structure use the periodic table to find the tota. In the phosphorus tribromide the central atom is P and is forms three sigma bonds with each bromine atom respectively.
This homework is due tomorrow by 8am. There are a total of 26 valence electrons for PBr3. Which is its molecular geometry.
Is the molecule polar or non polar. Which is its electron geometry. What is the electron geometry and shape of the molecule.
It has sp3 Hybridization and the bond angle is approximately 1095. Public health information CDC Research information NIH SARS-CoV-2. Is this molecule polar or.
QUESTION 1 10 points Save Answer What is the expected bond angle of PBr3. Draw the lewis structure of nitrogen tribromide. What is the formal charge bonding or non bonding.
1200 QUESTION 2 10 points Save Answer The electron geometry and the molecular geometry of SCl2 are respectively. Therefore the Bromine atom has three electron lone pairs around itself and one bond with hydrogen. There has been one report of PBr3 effectiveness on real-scale flames indicating an effectiveness two orders of.
A step-by-step explanation of how to draw the Br3 - Lewis Dot Structure Tribromide ionFor the Br3 - structure use the periodic table to find the total num. The molecule is trigonal pyramidal-shaped and is a polar molecule. The lewis structure follows the octet rule that states that for a molecule to be stable which states that there should be eight electrons in the outer shell of the atom for a molecule to be stable.
What is the hybridization of the central atom in the ion. Phosphorus halides including phosphorus trichloride and phosphorus tribromide have been considered as potential replacements for Halon 1301 as fire suppressants. There is 26 total valence electrons.
Draw the lewis structure of nitrogen tribromide. Here the s orbital of hydrogen combines with the 4p orbital of bromine. In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom.
An explanation of the molecular geometry for the IBr3 ion Iodine tribromide including a description of the IBr3 bond angles.

What Is The Molecular Geometry Of Pbr3 Enter The Molecular Clutch Prep

Video Lewis Dot Structure For Pbr3
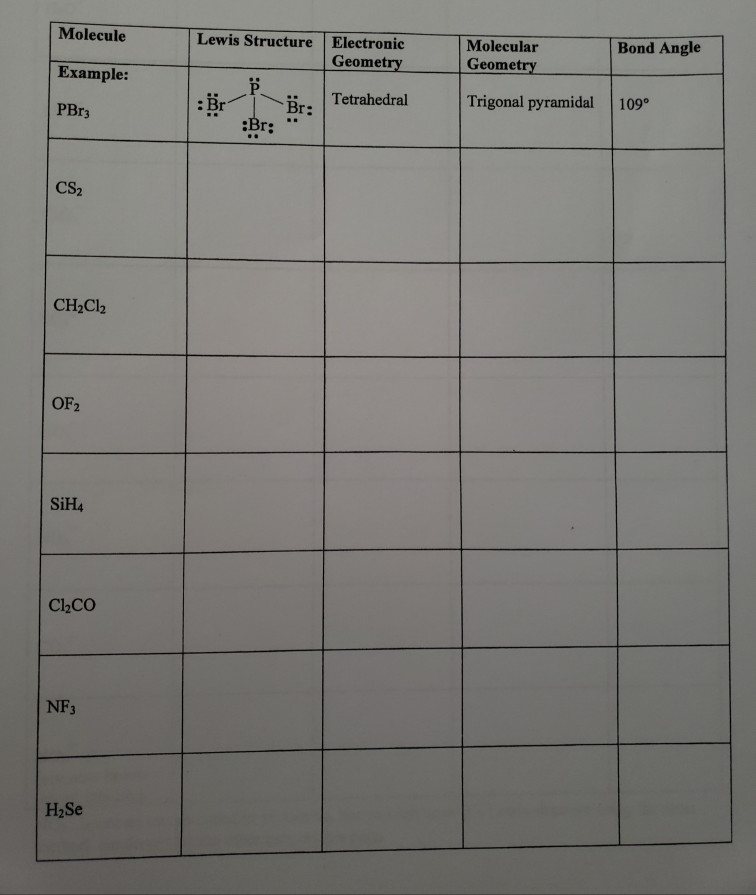
Moleculel Example Pbr3 Bond Angle Lewis Structure Chegg Com

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Https Www Lcps Org Cms Lib Va01000195 Centricity Domain 12702 Notes 20unit 204 20 20bonding 20ii 20key Pdf

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube
Is Pbr3 Polar Or Non Polar Quora

Pin By Sarah Hawkins On Chemistry Class Molecular Geometry Teaching Chemistry Chemistry Class

What Is The Molecular Geometry Of Pbr3 Enter The Molecular Clutch Prep
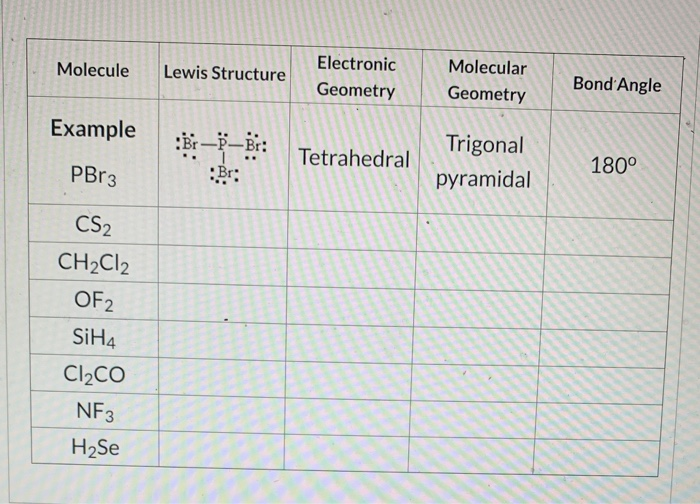
Calculate The Formal Charge Of Each Of The Molecules Chegg Com

What Is The Expected Bond Angle Of Pbr3 A 120 Degrees B 90 Degrees C 180 Degrees D 109 5 Degrees Study Com
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Chapter 6 The Shape Of Molecules Ppt Download

What Is The Molecular Geometry Of Pbr3 Enter The Molecular Clutch Prep

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Molecular Geometry Shape And Bond Angles Youtube

What Is The Lewis Structure Of Pbr3 How Is It Determined Quora