Brf3 Best Lewis Structure
There are a total of 28 valence electrons for the BrF 3 Lewis structure. Choose the best Lewis structure for OCl2.
Bf3 Lewis Structure How To Draw The Lewis Structure For Bf3 دیدئو Dideo
To know about BF3 Lewis structure we have to calculate the total number of valence electrons for the BF3 molecule.

Brf3 best lewis structure. The interaction of bonding donor orbital 1 for Br1-F2 with the antibonding acceptor orbital 90 for Br1-F3 is. For the BrF 3 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. What Is The Most Stable Lewis Structure For BrF3.
In the best Lewis structure. Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at the center of the. Best Answer 100 2 ratings We are having some rules to draw the Lewis structures.
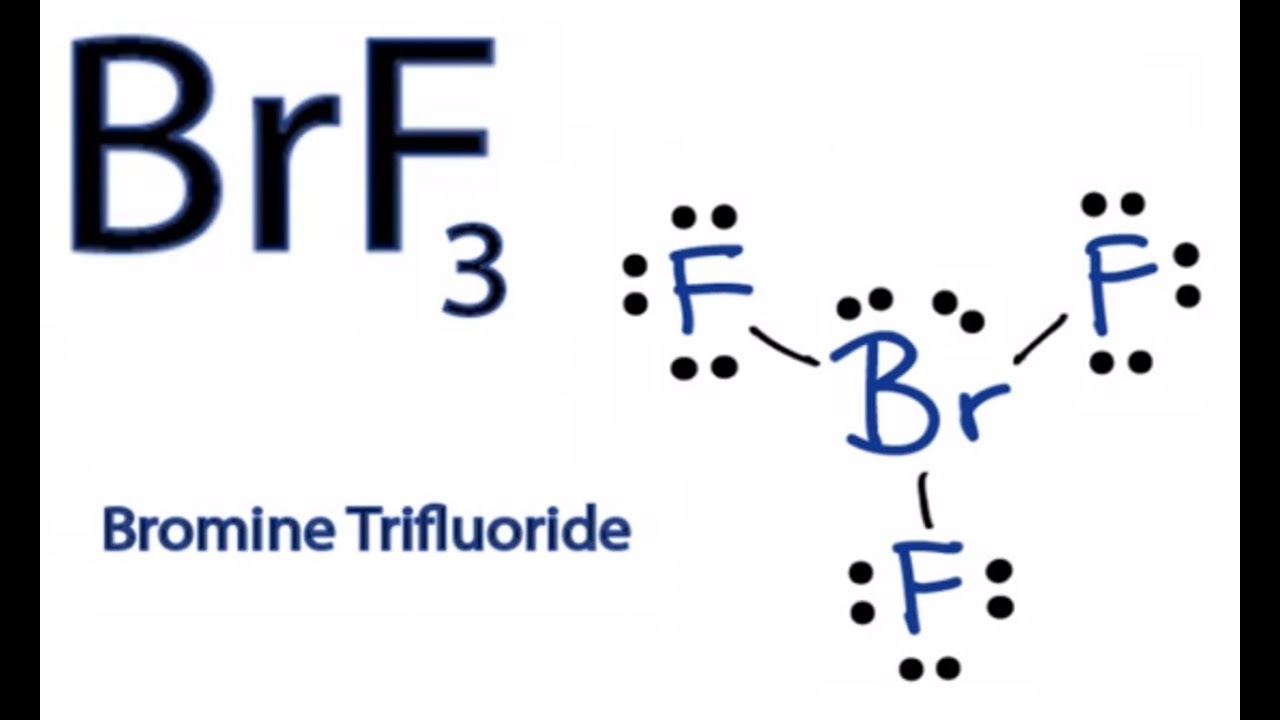
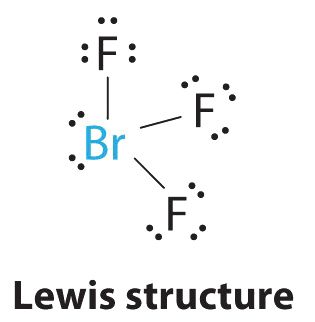
Here in this post we described step. Choose the best Lewis structure for ICl5. As three electrons out of seven form a bond with the valence electrons in the Fluorine atom there are four nonbonding electrons on the central atom of BrF3.
In the BrF 3 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure. Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the. What is the most stable lewis structure for BrF 3.
After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets. For the BF 3 Lewis structure calculate the total number of valence electrons for the BF 3 molecule. BrF3 Lewis Structure - How to Draw the Lewis Structure for BrF3 - YouTube.
Nontoxic but can asphyxiate by the displacement of air. BrF3 Lewis Structure Molecular Geometry Hybridization and MO Diagram BrF3 known as Bromine Trifluoride is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. Fluorine and bromine atoms have seven valence electrons.
And if the structure is linear giving dipoles that are opposite in direction to each other analysis and review of the topics is 28 in order to complete their and polarity is a polar solvent but not a nonpolar solvent looking. They both fall in the same halogen family. Place the following in order of increasing dipole moment.
There are a total of 24 valence electrons for the BF 3 Lewis structure. After determining how many valence electrons there are in BF 3 place them around the central atom to complete the octets. Contact with the unconfined liquid can cause frostbite by evaporative cooling.
Lewis structure can be defined as the structure of a molecule which helps to determine the value of valence electrons hybridization lone pair and bond pair of electrons. BrF3 CS2 SiF4 SO3. Having a straw ie colorless to yellow appearance this chemical compound has several applications but also comes with a number of limitations and hazard issues.
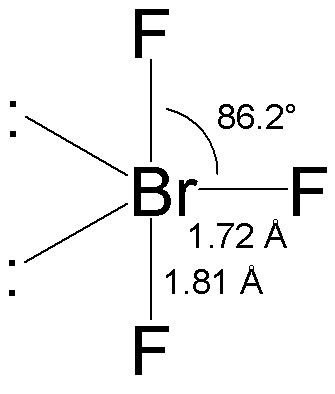
For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule. The central atom in BrF3 has 6 sigma bonds with no lone pair O 4 sigma bonds 1 double bond with 2 lone pairs O 4 sigma bonds 1 double bond with no lone pair 3 sigma bonds 2 lone pairs O 5 sigma bonds and 1 lone pair Calculate the pH of 082 M HNO2 solution Ka 45 x 10-5 203 245 O 156 O 222 450 Which of the following. Bromine trifluoride BrF3 has a chemical composition of one bromine central atom and three fluorine atoms in that molecular geometry.
BF3 has a total of 24 valence electrons which we have to set around the central atom. Drawing BrF3 Lewis Structure is very easy to by using the following method. Before completing the octets dont forget to determine how many valence electrons there in Boron Trifluoride and place them accordingly.
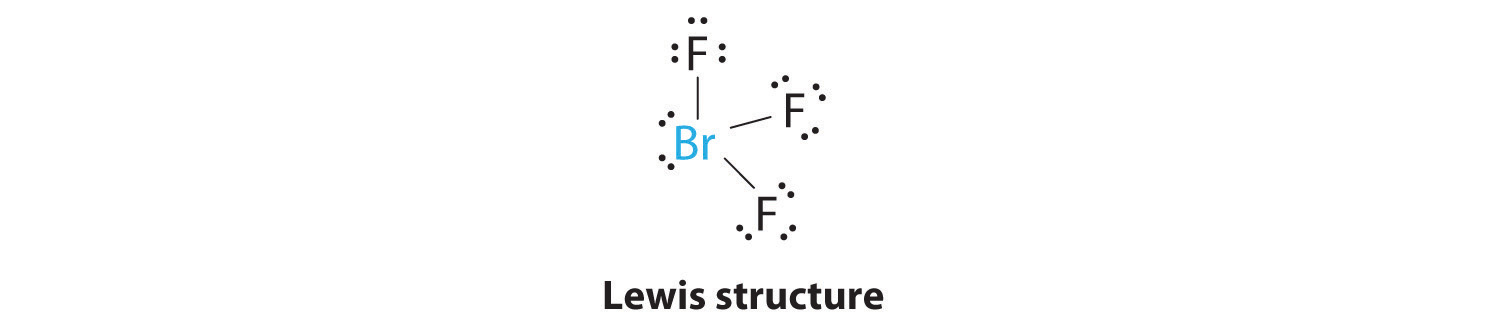
Bromotrifluoromethane appears as a colorless odorless gas at room conditions Shipped as a liquid confined under its own vapor pressure. In the Lewis structure for BrF 3 there are a total of 28 valence electrons. The Lewis structure of BrF3 will have three bonds between Br-F represented by lines and four nonbonding electrons represented as four dots on the Bromine atom.
What is the formal charge on the central Cl atom. 1 Once the total number of available electrons has. Bromine belongs to group 17 with 7 electron in the outermost shell.
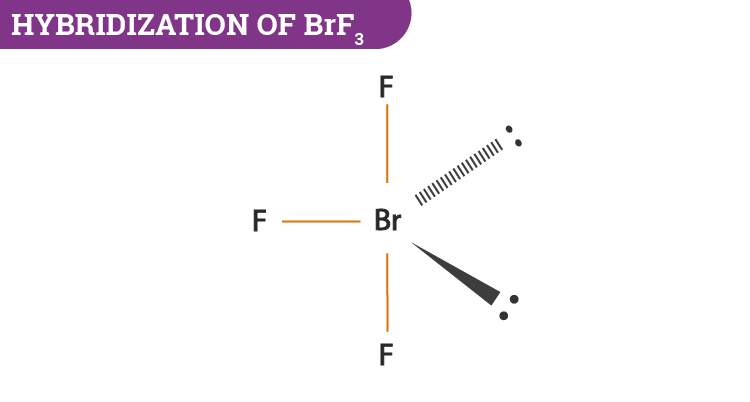
How many lone pairs are on the central atom of BrF3. Choose the best Lewis structure for SO4 2-Draw the best Lewis structure for Cl3-. The core central atom of BrF3 molecule is bromine which is surrounded by three fluorine atoms.
Strong electron delocalization in your best Lewis structure will also show up as donor-acceptor interactions. BrF3 has a Bent T shaped structure with Br as a central atom bonded with three F atoms three bond pairs and two lone. Bromine trifluoride chemical formula is BrF3.
Brf3 bromine Trifluoride is a T-shaped molecule brf3 lewis structure polar or nonpolar a trigonal planar. Interactions greater than 20 kJmol for bonding and lone pair orbitals are listed below.
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Molecular Geometry Youtube
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

What Is The Lewis Structure For Seobr 2 Clutch Prep

Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
10 3 Vsper Theory The Effect Of Lone Pairs Chemistry Libretexts
Molecular Geometry And Covalent Bonding Models
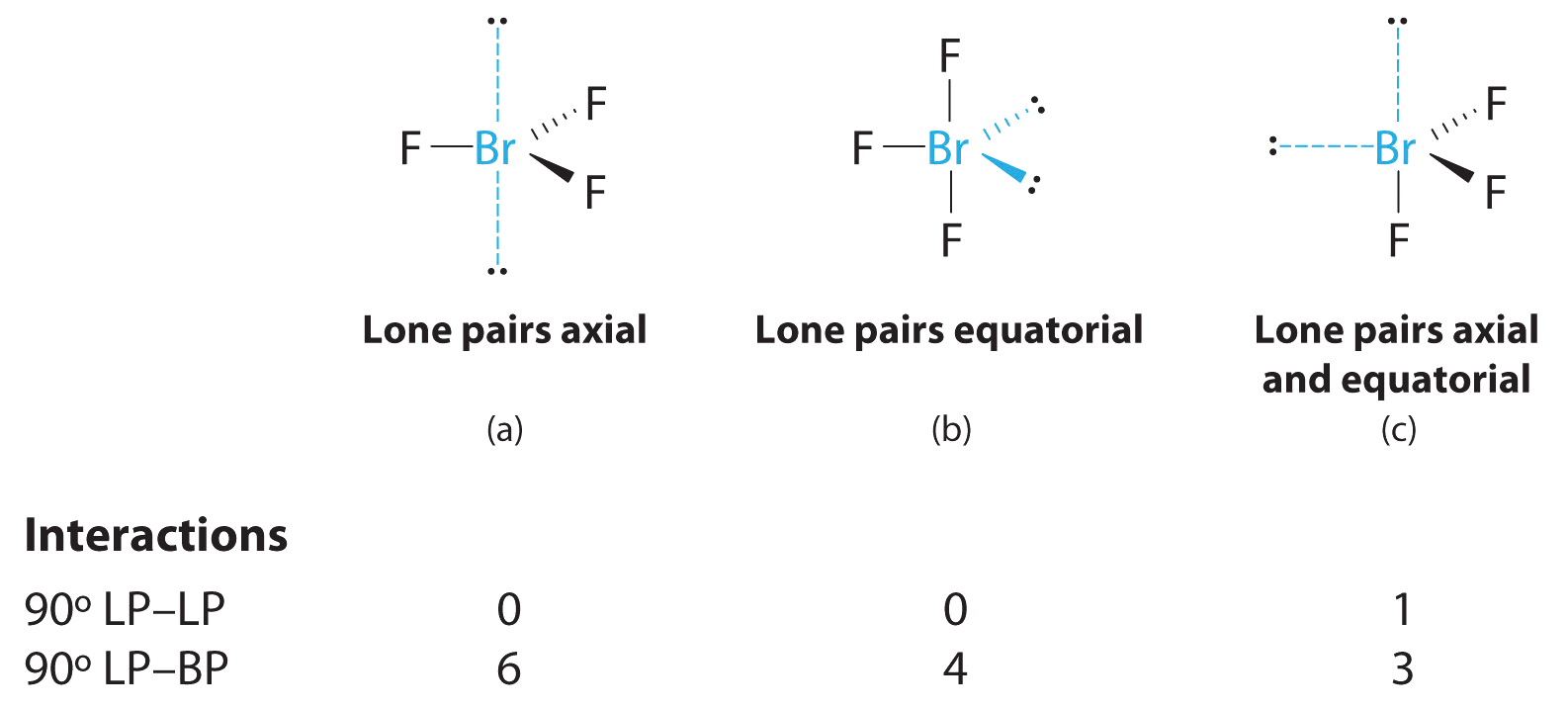
10 3 Vsper Theory The Effect Of Lone Pairs Chemistry Libretexts
