Draw The Structure Of H2o2 In Gaseous State
In gaseous phase the water molecule has a bent form with a bond angle of 1045. Thus water molecule has a bent structure.
Compare The Structure Of H2o And H2o2 From Chemistry Hydrogen Class 11 Cbse
The OH bond length is 957 pm.

Draw the structure of h2o2 in gaseous state. The H O bonds make an angle of about 1015 with the O O bond as shown. H 2 O 2 has a non-planar structure. Draw the gas phase and solid phase structure of H2O2.
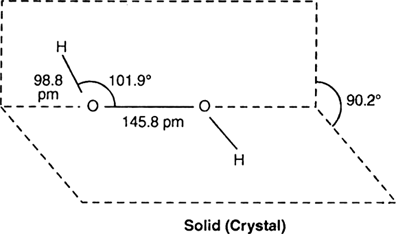
The structure of hydrogen peroxide in the gas phase and the crystalline state. The dihedral angle in gas and solid phase is 1115 and 902 respectively. Ii H 2 O 2 is a better oxidizing agent than water.
It is a colourless hygroscopic solid which can dissolve in many polar solvents. X is the structure of H 2 O 2 in gas phase and Y in solid phase X is the structure of H 2 O 2 in the gas phase and Y in the solid phase. Asked Mar 8 2018 in Class XI Chemistry by rahul152 -2838 points i Draw the gas phase and solid phase structure of H 2 O 2.
But below 1200 K it exists as dimer even in the vapour state. The rearrangement of ammonium cyanate to urea in aqueous solution at 50 c. The hydrogen atoms are placed one on each cover.
Although nonflammable it is a powerful oxidizing agent that can cause spontaneous combustion when it comes in contact with organic materialHydrogen. What is the structure of H2O2. The OH bond length is 957 pm.
Hydrogen peroxide has a non-planar structure both in gas and solid phase. Small amounts of gaseous hydrogen peroxide occur naturally in the air. The dihedral angle in gas and solid phase is 1115 and 902 respectively.
Hydrogen peroxide is unstable decomposing readily to oxygen and water with release of heat. The dihedral angle in gas and solid phase is 902o and 1115o. The O O linkage present between two oxygen atoms is called peroxide linkage.
Structure of H2o2 in gaseous state. In gaseous phase water molecule H2O has a bent form with a bond angle of 1045. Structure of H2o2 in gaseous state - 8979532 at317184 at317184 24032019 Chemistry Secondary School answered Structure of H2o2 in gaseous state 2 See answers.
O O single bond length is higher in the gas phase. The dihedral angle is 111. Thus the structure of H 2 O 2 is like an open book.
Structure of Hydrogen Peroxide. Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste. It has a skewed structure with a dihedral angle of 1115 gas phase which minimises repulsion between the lone pairs and the O-H bond pairs.
In vapour state above 1200 K it exists as a monomer having linear structure and zero dipole moment. The O O part of the molecule can be thought of as lying on the spine of a book open at an angle of 90. H 2 O 2 has an open book structure with O O spins.
But below 1200 K it exists as dimer even in the vapour state. The molar mass of the compound is 772 gmol -1. Ii The structure of H 2 O 2 is like that of an open book.
The OO linkage is called peroxide linkage. Draw a schematic diagram indicating the shape of the molecule clearly. The structure of H2O can be shown as.
STRUCTURE OF H2O2 H2O2 has a none planar structure in which two H atoms are arranged in two directions almost perpendicular to each other and to the axis joining the two oxygen atoms. Asked Feb 17 2020 in Chemistry by SurajKumar 662k points. Figure 16 the three most common states or phases of matter are solid liquid and gas.
And you will see that both oxygen atoms have two pairs of unbound electrons each. The structure can be shown as. The O-O bond length is 1458 pm and the O-H bond length is 988 pm which is equal to 988 10 -13 m.
This brings into effect the valence shell electron repulsion theory. In the solid phase the dihedral angle is reduced to 902 degree from 1115 degrees in the gas phase. Hydrogen peroxide has a non-planar structure both in gas and solid phase.
Structure Of H2O2 In Gas And Solid Phase. BeCl 2 is the formula of beryllium chloride which is an inorganic compound. Consider a process where a pure substance starts as a solid.
The O H bonds are in different planes. It is used in the manufacture of chemicals in metallurgy and. The structure of hydrogen peroxide is non-planar.
Draw a schematic diagram indicating the shape of the molecule clearly. Structure of the Molecule If you look at the dot diagram of H2O2 you will see the O-O bond.

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Lithium Element How Lithium Ion Batteries Work Use Of Lithium Chemistry Element Lithium Ion Batteries

Determining Hybridization Chemistry Organic Chem Science

Preparation Of Diethyl Ether Diethyl Ether Ethereal Ethyl Ether

Atom Model How To Make An Oxygen Atom Model With Thermocol School Project The4pillars Youtube Atom Model Carbon Atom Model Atom Model Project

Chemistry Pals Laboratory Preparation Of Nitrogen Ii Oxide Chemistry Saved Pages Nitrogen

H2o2 Structure Molecular Chemistry Inbox Screenshot

Simple Procedure For Writing Lewis Structures For Methanimine Ch2nh 25 Chemistry Net Writing Chemistry Math Equations

Dalton S Atomic Theory Atomic Theory Gcse Science Nuclear Physics

The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Tech Company Logos Molecules

Students Use Marshmallows And Toothpicks To Develop Models Of Molecular Structures In Addition Students Utilize G Molecule Model Molecules Teaching Chemistry

The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Tech Company Logos Molecules

The Chemical Equation For The Decomposition Of Hydrogen Peroxide In The Presence Of A Cat Chemical Equation Oregon Health And Science University Peroxide Water

Structure Of Diamond And Graphite Beach Jewelry Diy Diamond Delicate Jewelry Necklace

Chemistry Pals Structure Of Hydrogen Flame Chemistry Hydrogen Gas Flames

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules