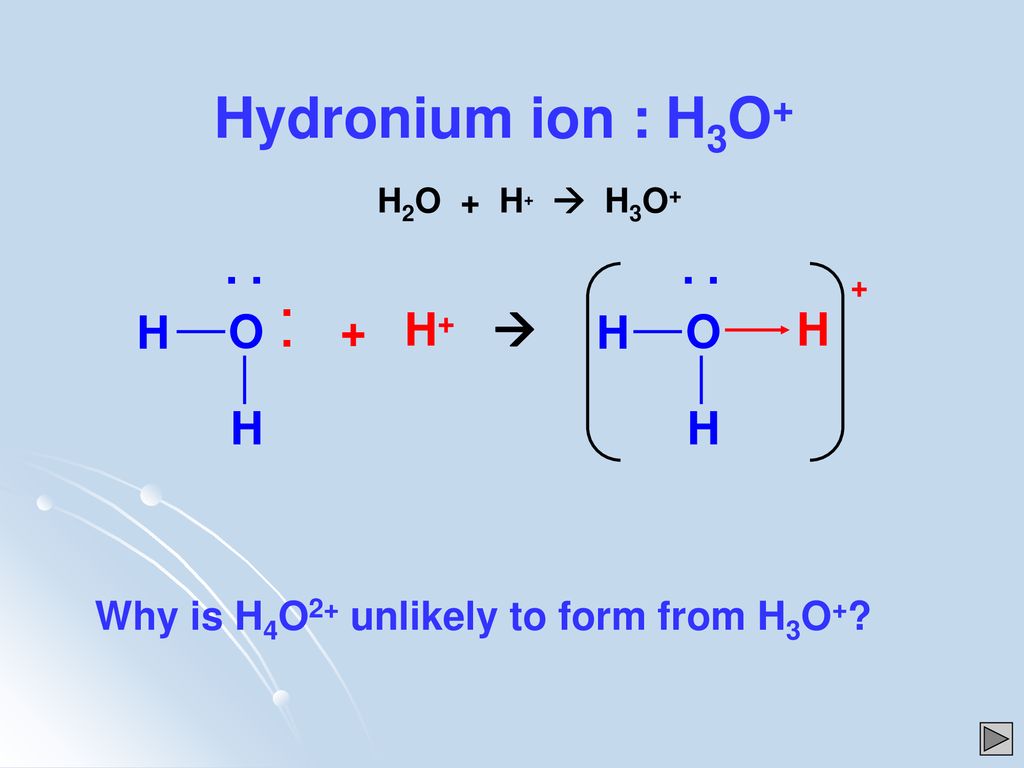
Does H3o+ Have Resonance Structures
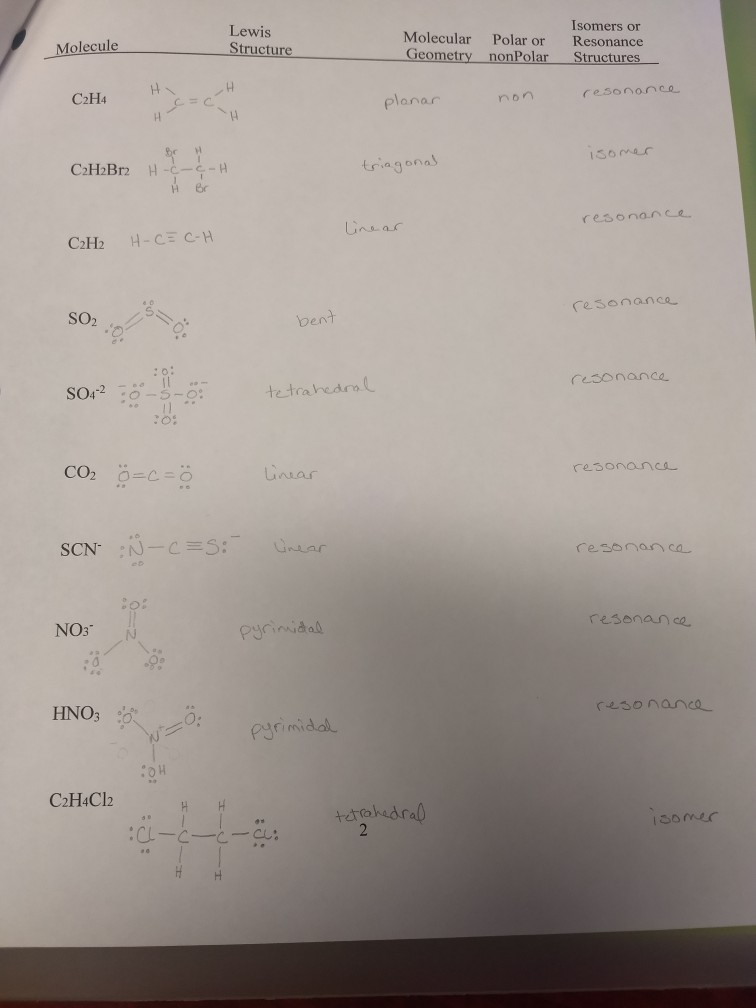
It is helpful if you. C2H4 and C2H2Br2 I need help with these compounds as to whether or not they have isomers.
What Is The Shape Of H3o Ion Quora
I have to draw the isomers or resonance structures.

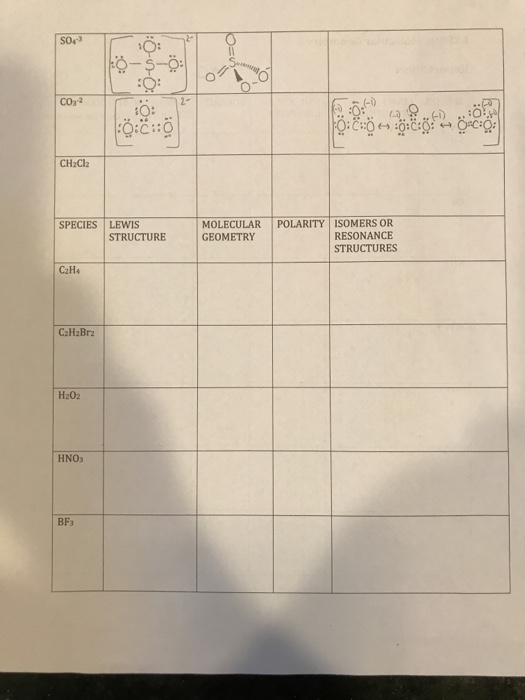
Does h3o+ have resonance structures. Such a structure would be super unstable very high energy that it wont exist. Therefore we only have 8 valence electrons for the H3O Lewis structure. I dont know if H2O H3O or SO4 3- have any and I cant find them.
Answered 1 year ago Author has 21K answers and 5656K answer views. A molecule or ion with such delocalized electrons is represented by several contributing structures also called resonance structures or canonical forms. I have seen in my textbook that.
Try to draw the H 3 O Lewis structure before watching the video. The structure O-NCl has formal charges of -1 on O and 1 on Cl. When it is possible to write more than one equivalent resonance structure for a molecule or ion the actual structure is the average of the resonance structures.
H3O is an important compound in Acid-Base chemistry and is considered an acid. Former Professor of Chemistry at Academic Consulting. Any molecule in which all of the bonds are identical will be nonpolar.
For the H3O Lewis structure we first count the valence electrons for. Ozone or O3 has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. They all have resonance structures.
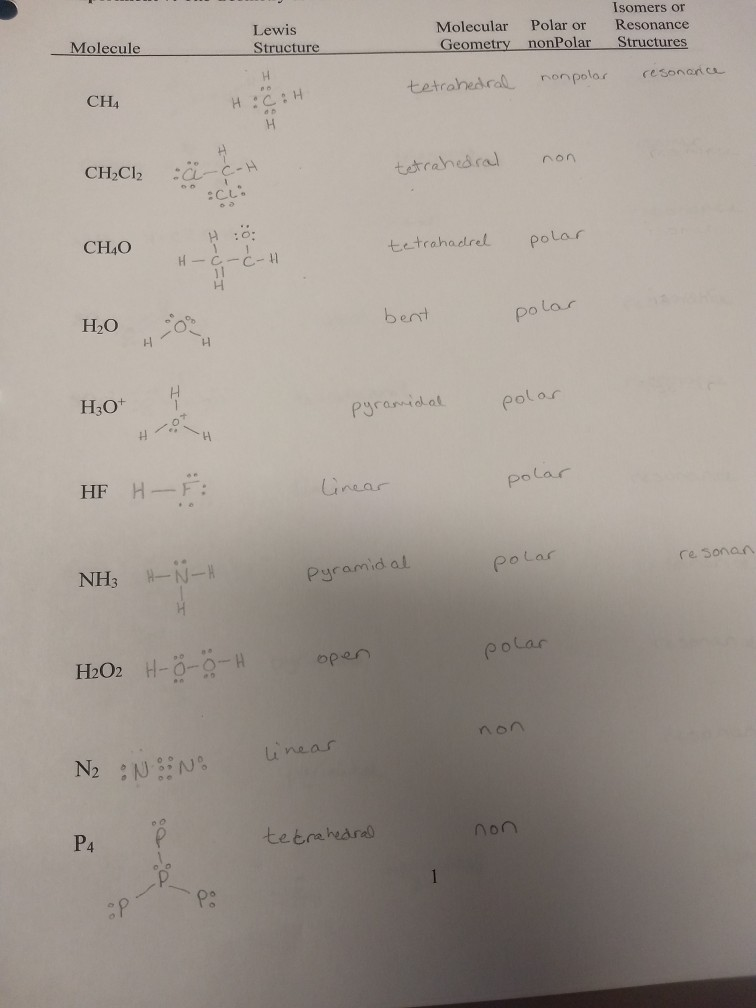
After all its much easier to form ON-Cl why would we ever want O-NCl. CH4 CH2Cl2 CH4O H2O HF NH3 I have these compounds listed as having isomers. Is H3O A Sp3.
The central atom for H 3 O is O since Hs cant be a central atom. Both structures account for the needed 18 valence electrons - 6 from 3 bonds and 12 as lone pairs placed on the. A double headed arrow on both ends of the arrow between Lewis structures is used to show resonance.
Which of the following is the correct Lewis Structure for the Hydronium Ion H3O. Resonance structures must also have the same number of lone pairs. I have the following compounds listed as having no isomers.
CH4O tetrahadrel polar H-C H НО bent polar H3O Pyramidal polar H-F. H H CH2C12 tetrahedral non H0. Oxonium is an oxygen hydride and an onium cation.
Pick the Correct Arrow for the Job Most arrows in chemistry cannot be used interchangeably and care must be given to selecting the correct arrow for the job. This species has its three atoms bonded sequentially in the following fashion. Describe the bonding in this species.
Also I think SO4 is suppose to be 2- not 3-. Isomers or Lewis Structure Molecule Molecular Polar or Geometry nonPolar Resonance Structures CH4 tetrahedral nonpolar resonanc. Methane does not has any resonating structures or isomers.
97 84 ratings FREE Expert Solution. Resonance occurs when we have two structures of similar energy resulting in electron delocalization. I have to draw the isomers or resonance structures.
Assume that bonding follows the octet rule. These structures are written with a double-headed arrow between them indicating that none of the Lewis structures accurately describes the bonding but that the actual structure is an average of the individual resonance structures. A step-by-step explanation of how to draw the H3O Lewis Structure Hydronium Ion.
Does h3o have resonance structures. The Carbonate CO_32 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Draw a Lewis structure for H3O Show all unshared electrons and the formal charges if any.
N2 C2H2 and CO2. I dont know if H2O H3O or SO4 3- have any and I cant find them. I have seen the structure of triiodide ion I X 3 X but I cannot understand why this structure is even possible.
Attached it what I have so far. HF Linear polar HIPIS NH3 Pyramid al Polar re sonan H2O2 H- polar open non near N2 N N P4 tetrahearas non op -1 Lewis Structure. O has 2 bonding electrons and 2 lone pairs therefore a neutral oxygen.
Watch the video and. It accommodates electrons in empty d orbitals. I X 3 X is formed by combination of I X 2 and I X ion in which I X ion acts as a donor and I X 2 molecule act as acceptor.
It is a conjugate acid of a water. Draw your resonance structures so that the atoms in them are bonded together in this order Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn.

H3o Lewis Structure Hydronium Ion Youtube

Electron Dot Structure Of Hydronium Ion Brainly In
As Chemistry Coordinate Or Dative Covalent Bonding Nh4 Al2cl6 Co H3o Ppt Download
Isomers Or Lewis Structure Molecule Molecular Polar Chegg Com

Draw The Lewis Structure For The Polyatomic Hydronium Chegg Com

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
I Have To Draw The Isomers Or Resonance Structures I Chegg Com

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

Chemistry Chemical Bonding 22 Of 35 Lewis Structures For Ions Hydronium Ion H3o Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

H3o Molecular Geometry Shape And Bond Angles Youtube
Isomers Or Lewis Structure Molecule Molecular Polar Chegg Com
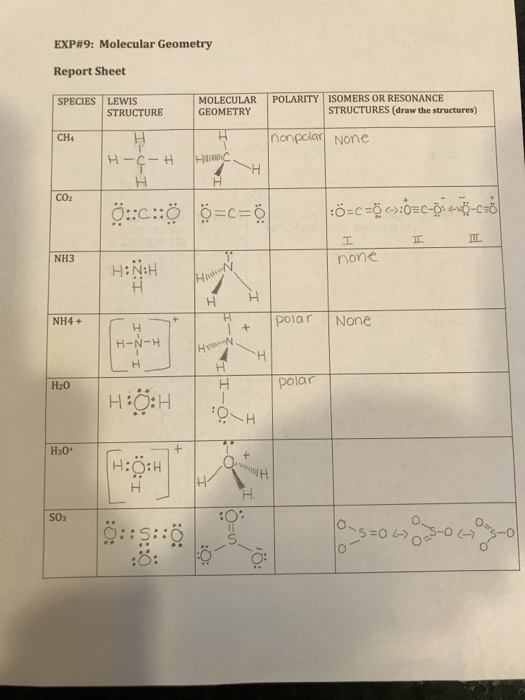
I Have To Draw The Isomers Or Resonance Structures I Chegg Com
What Is The Shape Of H3o Ion Quora
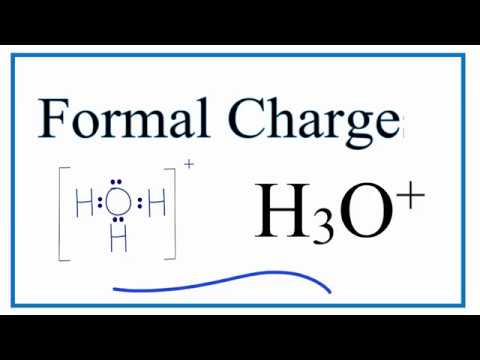
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube
