Does Xef2 Have Resonance
2 electron pairs - linear electron pair geometry It also failed to explain the geometry of complex molecules. Structural drawings of isomers represent separate molecules.
Freezing in Resonance Structures for Better Packing.

Does xef2 have resonance. This problem has been solved. Does xef2 exhibit resonance. December 10 2020.
Ii Because of small size of flourine atom and strong electron-electron repulsions in its compact 2p orbitals. Draw the Lewis structures for BrO3- and ClO4- and indicate their correct number of additional resonance structures. What hybridization does XeF2 have.
If z2 2 sp. Thus the hybridization of XeF2 molecule is sp3d. Resonance structures can also be non-equivalent in which case they will have different numbers andor locations of bonds.
Converting from one isomer to another requires breaking σ sigma bonds and forming new σ bonds. Does xef2 exhibit resonance. Pairs of dots are used to represent lone pair electrons.
What I am thinking. But as Xenon does not form bonds easily this compound is an exceptional case. It is helpful if you.
ICl4- Yes COF2 Yes TeCl4 no XeF2 no 5248 results Chemistry. Examples are shown for the molecules SF2 and CH2O below. If there are more electrons than it then that Here the steric number for the central Xenon atom is 5.
Im a little confused about this. What I am thinking. What Is Its Molecular Geometry.
In this case they do not exceed the octet rule. Does XeOF2 Have Resonance Structures. Do ICl4- COF2 TeCl4 XeF2 have possible resonance structures.
Pairs of dots are used to represent lone pair electrons. 1 The impedance of a parallel L-C circuit is low at resonance and higher at frequencies above and below resonance. Try to draw the XeF 2 Lewis structure before watching the video.
XeF2 Becomes XeFF at Large Compression. Examples are shown for the molecules SF2 and CH2O below. The molecule SO2 shown above has two such resonance formsResonance structures can also be non-equivalent in which case they will have different numbers andor locations of bonds.
Used as a very conve. SnCl2 H2O are angular as the centra view the full answer. And has an bond angle of 180o.
The bond angle of F-Xe-F is 180 degrees. I so much appreciate thisthrough this i could deferentiate compound. Remember that Xenon can have more than 8 valence electrons.
I expect structure 3 to be rare because of the high. Do ICl4- COF2 TeCl4 XeF2 have possible resonance structures. Any extra bonds are ionic in character with the extra electrons assigned to the outer atoms.
Resonance structures represent the same compound while isomers are chemically distinct. CO2 SnCl2 H2O XeF2 Of the given compounds CO2 is linear. Does XeOF2 have resonance structures.
There is an EASY way and a FORMAL way to draw the Lewis structure of ClF 3. Z Number of Valence electrons on central atom number of monovalent groups attached - charge with sign Now divide Z by 2. I like using a formula I learned in high school in India.
Xe F 2 XeF 2. Sometimes one dot structure is not enough to completely describe a molecule or anion sometimes you need two or more and heres an example this is the acetate anion and this dot structure does not completely describe the acetate anion we need to draw a resonance structure another resonance structure and so what were going to do is take a lone pair of electrons from this oxygen and move that. Which of the following compounds have resonance structures.
Resonance structures are different approximate representations of the same molecule. Hence the hybridization of the central atom Xe is sp3d. It is a powerful fluorinating as well as an oxidizing agent.
The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Generally the Lewis structure is helpful to understand the molecular geometry of any given chemical compound. With this model we can draw a series of resonance.
Hydrogen has 1 valence electron we have 2 Hydrogens. Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel. Consider the resonance structures you drew above and what you know about basicity.
An alternative view is that stable hypervalent molecules have only four covalent bonds. The molecule SO2 shown above has two such resonance forms.

Is O3 Polar Or Non Polar Ozone In 2021 Ozone Chemical Formula Polar

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules
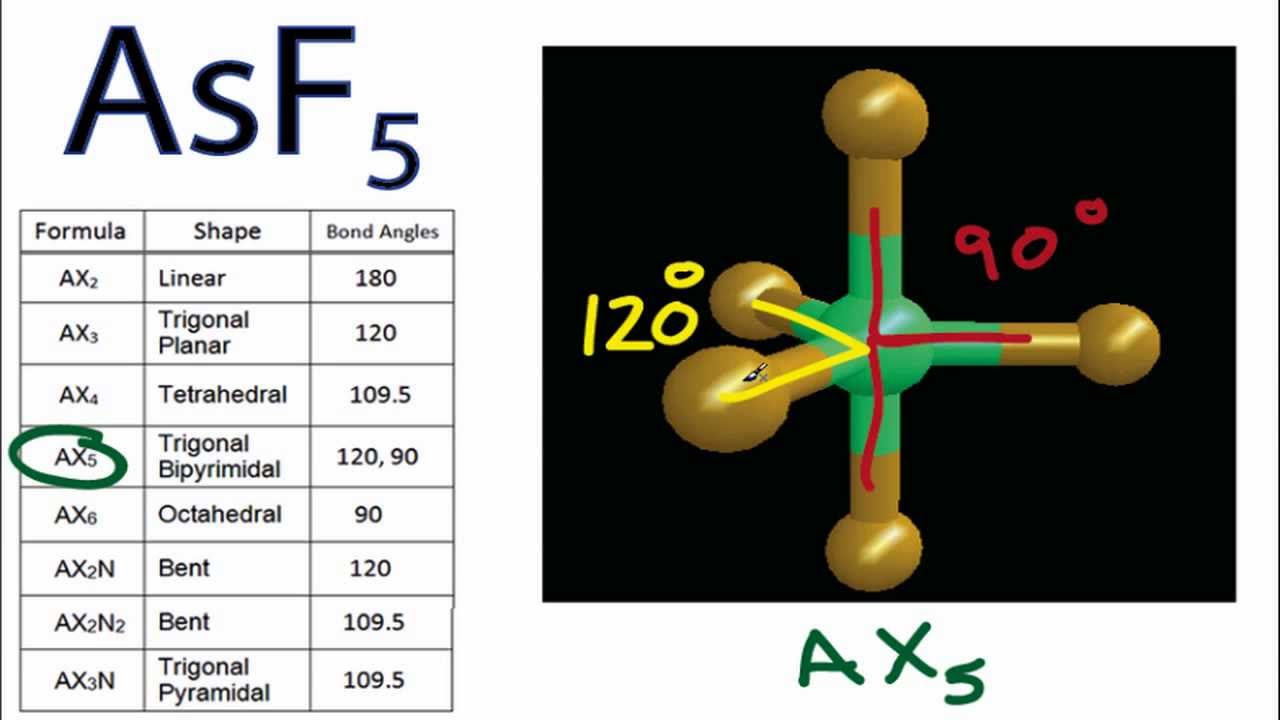
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules
