How Many Valence Electrons Does Ni3 Have
How Many Protons Neutrons And Electrons Does Argon Have. Nickel Ni palladium Pd platinum Pt and darmstadtium Ds.

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
And so forth up to group 18.

How many valence electrons does ni3 have. Videos you watch may be added to the TVs watch history and influence TV. How Many Valence Electrons Does Nickel Have. Both of the Bromine atoms have octets they have 8 valence electrons.
Answer Magnesium has just two valence electrons and 12 electrons. In order to know the full electronic configuration of lead it is important that the users must first understand what is meant by the term Lead Electron Configuration. It contains 2 electrons.
Lithium is called alkali metal. How Many Protons Electrons And Neutrons Are Found In Ethanol. These elements are transition metals.
After the electron configuration the last shell of the nitrogen atom has five electrons. Elements in group 13 have three valence electrons. Dec 28 2016 2.
That is the IBr2- Lewis structure. How many valence electrons does nitrogen ion have. In this regard can nitrogen have 8 valence electrons.
How many valence electrons does Ni have. Therefore no matter how electrons are shared between the nitrogen and oxygen atoms there is no. Helium does not participate in.
It has 18 electrons. Nitrogen has a total of 5 valence electrons so doubling that we would have a total of 10 valence electrons with two nitrogen atoms. How many valence electrons does californium have.
By signing up youll get thousands of step-by-step solutions to your homework. Fluorine has 9 electrons 2 in the first shell and 7 in the second shell so seven valence electrons. The valence electrons of helium are two and the total number of electrons is two.
Therefore Ni has 2 valence electrons. Magnesium is classified as a metal. They have between 3 and.
Nickel has 2 electrons in its valence fourth shell and can lose 2 electrons or gain 6 more electrons to make it stable. How many valence electrons does nickel have. Get more help from Chegg.
Lithium is the element of group-1 and the symbol is Li. How many valence electrons does an ca2 ion have. Metals are the elements which have a tendency to loose electrons and thus they form cations.
Look at the Periodical Table. In this case the valence electrons of nitrogen are 5. Thus all atoms of this element have 17 protons and 17 electrons.
The formula of ethanol is C2H5OH. 1 Answer Douglas K. It can also be written as EC so as to save time for students.
We just add the valence electrons of each atoms involved. We know the details about this. NI3 is called Triiodoamine or nitrogen triiodide.
How do valence electrons affect chemical bonding. I dont understand. The Group 3 atoms have 3 valence electrons.
There are 2 types of elements in the periodic table. That means it can hold more than eight valence electrons. Elements in group 1 have one valence electron.
How many valence electrons does nitrogen have. The valence electron of lithium is one. Elements in group 14 have four valence electrons.
There are atomic orbitals in chemistry and the role of electronic configuration is that it tells us how many electrons. And the Iodine it has 10 but Iodine is in period 5 on the periodic table. The valence electrons which are there in the outermost shell of lead is 4.
Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electrons. Correspondingly how many valence electrons does IBr2 have. The Group 2 atoms have 2 valence electrons.
Sodium will loose 1 electron. The reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding. Full Electron Configuration For Lead.
Group 10 has the following elements. Carbon C has 6 protons and electrons and 6 neutrons hydrogen H. The symbol for the helium element is He.
After arranging the electrons it is seen that the last shell of the nitrogen atom has five electrons. The elements that have 5 6 or seven electrons in the last shell. No Nickel has 2 electrons in its outermost shell.
The total number of valence electrons is 5611. By Staff Writer Last Updated April 6 2020. First we need to count the total number of valence electrons.
Chemistry The Periodic Table Valence Electrons. Elements in group 2 have two valence electrons. For the NI 3 Lewis structure there are a total of 26 valence electrons available.
If playback doesnt begin shortly try restarting your device. In the same vein people ask how many valence electrons does group 10 have.

Ni3 Ipr2im 3 µ2 Co 3 µ3 Co A Co Stabilized Nhc Analogue Of The Parent Neutral Nickel Chini Type Cluster Berthel 2019 European Journal Of Inorganic Chemistry Wiley Online Library
Band Structure Dos And Schematic Filling Of The Valence Shell For Ni Download Scientific Diagram

Lewis Structures Covalent Bonds Hbr Scl2 Mgbr2 Pbr3 Ni3 Bf3

Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Youtube

Crystal Data And Structure Refinement Of Ni3 And Ni7 Download Scientific Diagram

How To Draw Lewis Structure Of Ni3 Drawing Easy

10 This Questions Below Deal With Structure Of Ni3 Chegg Com

A Xrd Patterns Of The Ni 3 Hitp 2 And Si Ni 3 Hitp 2 Composite Download Scientific Diagram

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Solved Draw The Lewis Structure For Ni3 How Many Valence Chegg Com

Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

What Is The Lewis Structure Of Ni3 Study Com

Normalized Ni K Edge X Ray Absorption Spectra Of The Ni 3 Al And Ni 3 Download Scientific Diagram
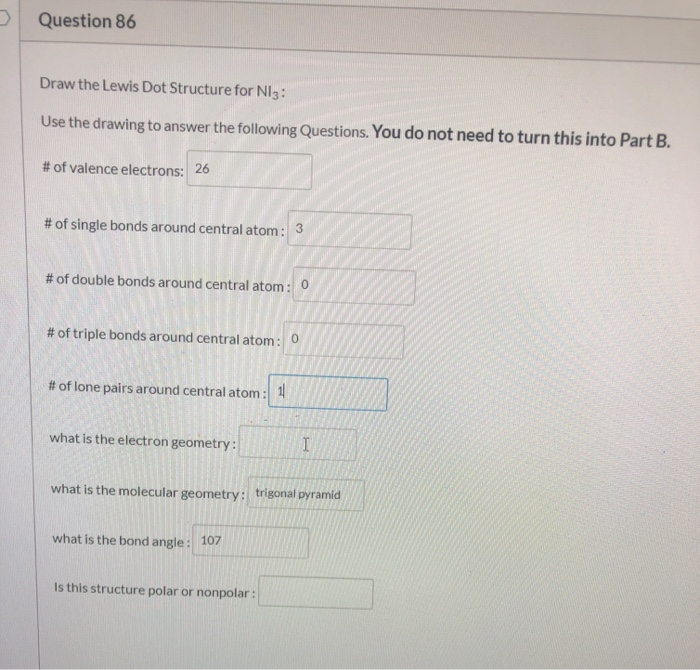
Question 86 Draw The Lewis Dot Structure For Ni3 Use Chegg Com
Solved Draw A Lewis Structure For Each Molecular Compound A Of2 B Ni3 C Cs2 D Course Hero

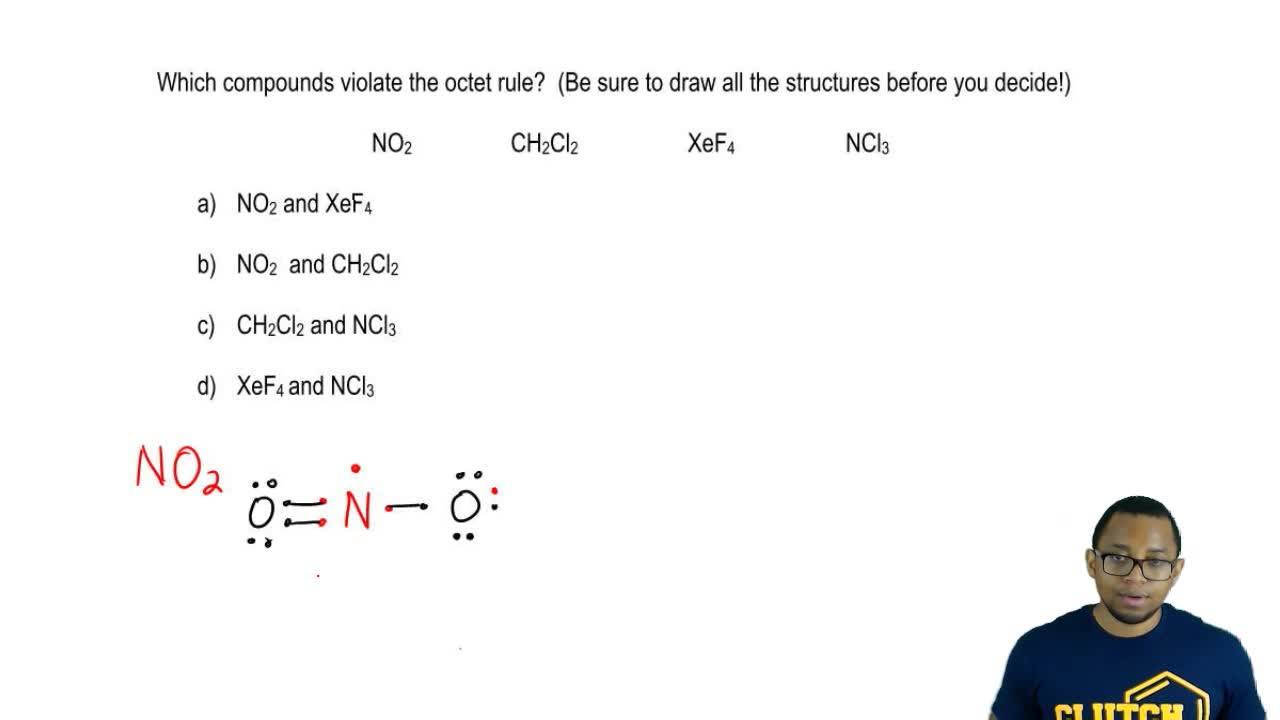
Answer Which Compounds Violate The Octet Clutch Prep
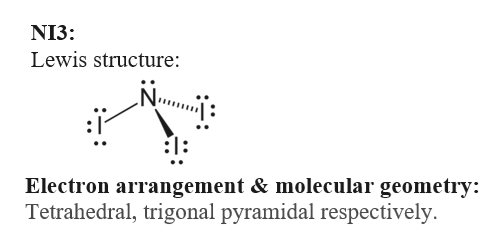
Answered Draw The Lewis Structure And Determine Bartleby