Lewis Structure If5
Each F is pulling the electron density of the bond to the Fluorine nucleus. IF5 Lewis Structure.

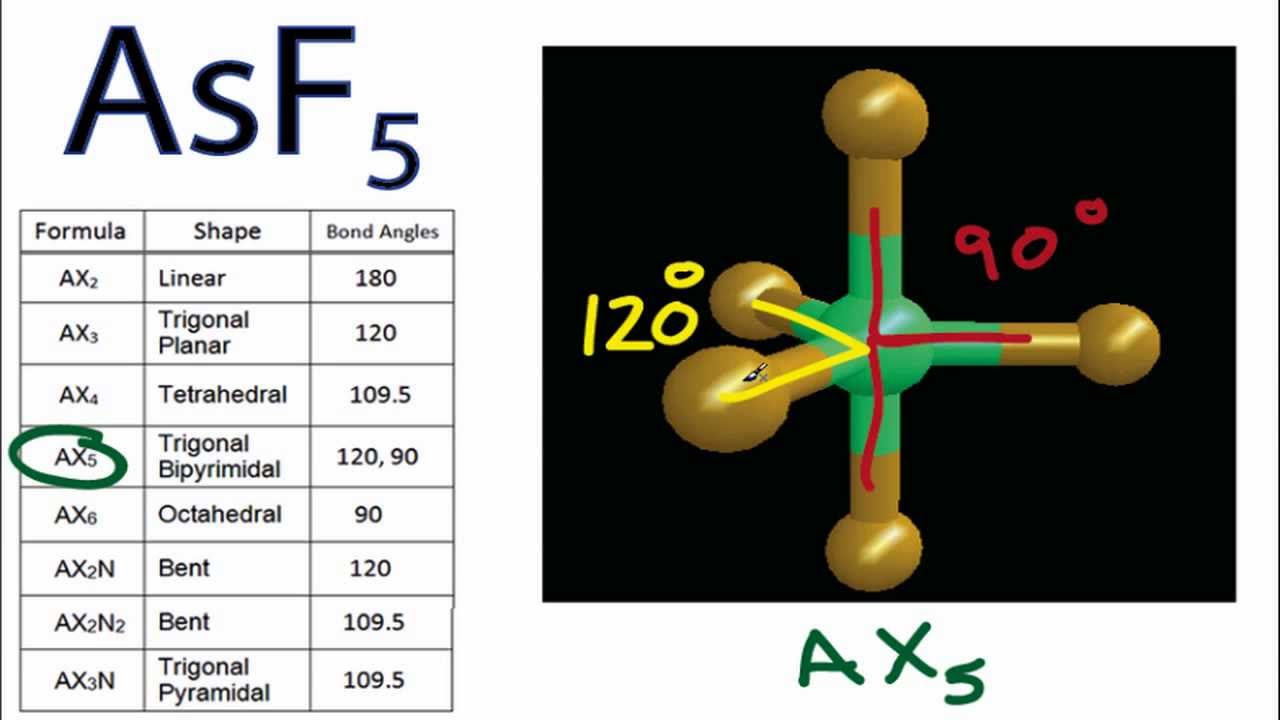
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry
There are polar bonds between the I and F.
Lewis structure if5. The two sets vertical and the one at top left-bottom right. A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure Bromine pentafluorideFor the BrF5 structure use the periodic table to find the tota. In the Lewis structure for IF5 youll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure.
Iodine pentafluoride is a colorless liquid at room temperature. A Lewis structure basically represents the number of valence electrons of an atom. What is the point group of IF5.
It is an interhalogen compound that is used as a fluorination reagent in organic synthesis. This leaves a pole from the I to the F. Steric Number - A focus into the key to the VSEPR Theory.
After determining how many valence electrons there are in IF5 place them around the central atom to. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. The IF 5 Lewis structure youll need to put more than eight valence electrons on the Iodine atom.
In the IF 5 Lewis structure Iodine I is the least electronegative atom and goes in the center of the Lewis structure. Iodine Forms A Series Of Fluorides. Notice that four of the bonds can cancel.
IF5 has the square pyramidal geometry with. The density of this molecule is around 3250 gcm3. Representative Metals Metalloids and Nonmetals.
How can Iodine bond with 5 Fluorines in Iodine. The above image is the geometrical structure of the Iodine pentafluoride molecule. If5 Lewis Structure Molecular Geometry.
This is the Lewis structure for IF5. Lewis Diagram If5 - wiring diagram fur-circular-b - fur. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
And for more detail you can also refer to the lewis structure of IF5. Melting Point- 943 C. Iodine forms a series of fluorides listed here.
Draw Lewis structures for IF5 and BF4- bartleby. Boiling Point- 9785 C. Remember that Iodine I can hold more than eight valence electrons.
Before we begin making the Lewis structure for IF5 there are a few things to keep in mind. How many lone pairs are there in IF5. In the Lewis structure for IF 5 there are a total.
IF5 Lewis Structure Hybridization Polarity and Molecular Shape I2 5F2 2IF5. A IF b IF3 c IF5. Polar bonds can cancel themselves if the geometry is correct.
Iodine pentafluoride F5I CID 522683 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. IF IF3 IF5. For the IF5 Lewis structure calculate the total number of valence electrons for the IF5 molecule.
The melting point of IF5 is 943 C or 4897 F. How many bonds can Silicon form. The easiest way lớn determine the number of valence electrons of an atom is by counting the number of columns of a periodic table left lớn right excluding the transition elements.
A step-by-step explanation of how to draw the IF3 Lewis Dot Structure Iodine trifluorideFor the IF3 structure use the periodic table to find the total num. Before we begin making the Lewis structure for IF5 there are a. Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule.
This creates polar bonds. 5 of them to be exact.

Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry