Lewis Structure Ni3
There are 24 valence electrons available for the Lewis structure for NO 3-. NI 3 is very similar to NH 3 and NF 3.

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Demonstrations will often use a feather to cause the explosion.

Lewis structure ni3. Ni3 Lewis Structure How To Draw The Dot For. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3-lewis structure. Ammonia is lighter than the air colorless and pungent in smell.
In many these spin states vary between high-spin and low-spin configurations Start by drawing the Lewis structure NI3. A stepbystep explanation of how to draw the ni3 lewis dot structure nitrogen triiodide. I also go over hybridization shape and bond angles.
Free unlimited access for 30 days limited time only. Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state.
NI 3 is highly explosive when dry. The shape of a NI3 molecule. Log in Sign up.
This problem has been solved. Ni3 lewis structure. Bond angle of a NI3 molecule.
What is the Lewis structure of NI3. Draw The Lewis Structure For NI3. Lewis Structure of NO 3-Nitrite ion Lewis structure of NO 3-ion is drawn step by step in this tutorial.
There are a total of 26 valence electrons in NI 3. Sncl3 Lewis Structure What is the molecular geometry of sncl3. CH 2 O f.
Terms in this set 14 the shape of an H2 molecule. What is the Lewis structure of NI 3. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds.
Drawing the Lewis Structure for NI 3. The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry. What is the Lewis structure of NI3.
Does ni3 have a lone pair. Nitrogen triiodide NI3 or I3N CID 61603 - structure chemical names physical and chemical properties classification patents literature biological activities. Bond angle of an H2 molecule.
The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair. Expert Answer NI3 5113 8 T IN L NI3is an instable compound the instability of NI3isattributed due to th view the full answer. H 2 O m.
Are molecular or coval. It is a metal cation allergen a nickel cation and a monoatomic trication. Nickel 3 is a nickel cation in which the nickel carries a triple positive charge.
The shape of an H2S molecule. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future. States when describing transition metal coordination complexes refers to the potential spin configurations of the simpler electron path of electron.
One of these is a non-bonding lone pair. When it explodes it produces a cloud of purple smoke this is iodine gas. Lewis dot structures for polyatomic ions this video shows how to draw ions.
What is the Lewis structure of NI 3. The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair. For structure calculate total number valence.
Bond angle of a CCl4 molecule. The nitrogen atom contributes 5 valence electrons. It is termed a contact explosive since it doesnt take much to set it off.
Draw the Lewis Structure for NI3. Get the detailed answer. Draw Lewis dot structures of NI 3 and NH 3 showing all the valence electrons and explain why the stabilities of these molecules are so different.
Free unlimited access for 30 days limited time only. Cl 2 r. Log in Sign up.
For structure calculate total number valence electrons the. SOLUTION a The Lewis structure of the SnCl3 ion looks like this. N 2 O i.
The shape of a CCl4 molecule. Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. Get the detailed answer.
NH3 Lewis Structure Geometry and Hybridization Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. The Lewis structure for NI 3 is. CF 2 H 2 e.
Bond angle of an H2S molecule. CCl 2 F 2 d.
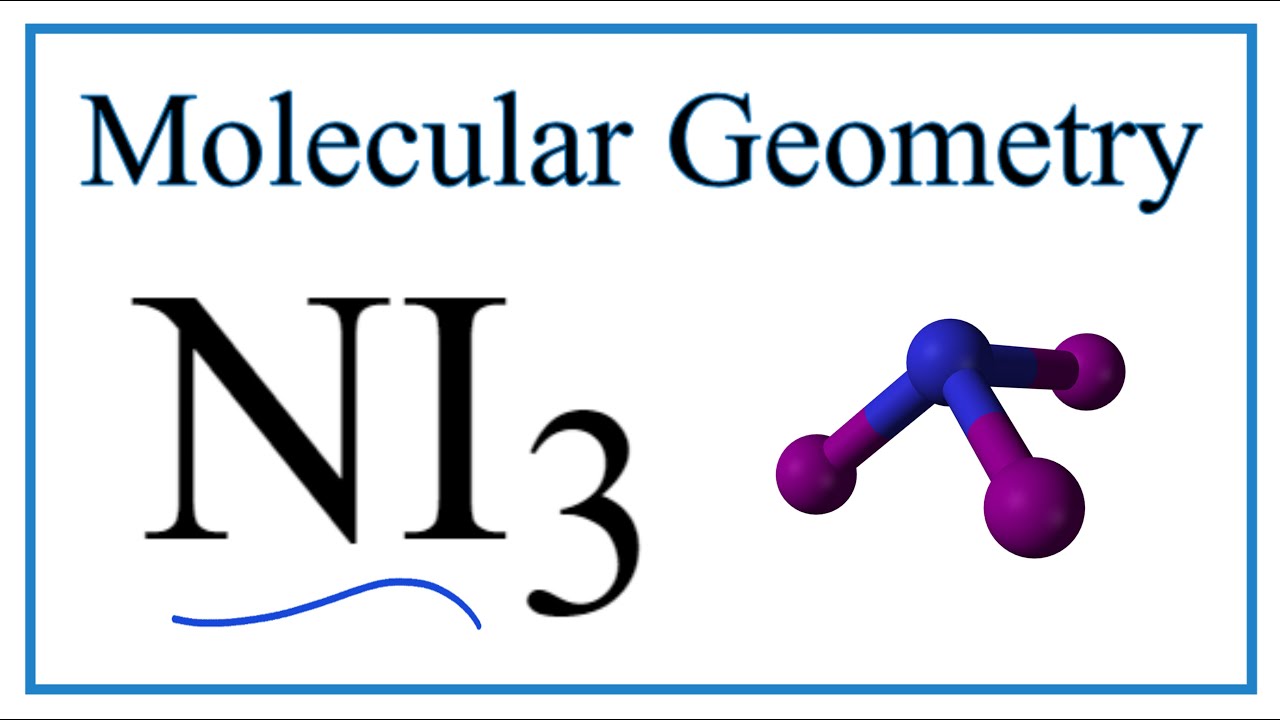
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
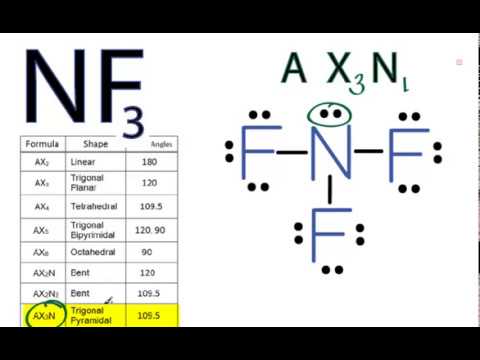
Nf3 Molecular Geometry Shape And Bond Angles Youtube

Wn Sbi3 Lewis Structure Molecular Geometry Bond Angle Polar Or Nonpolar
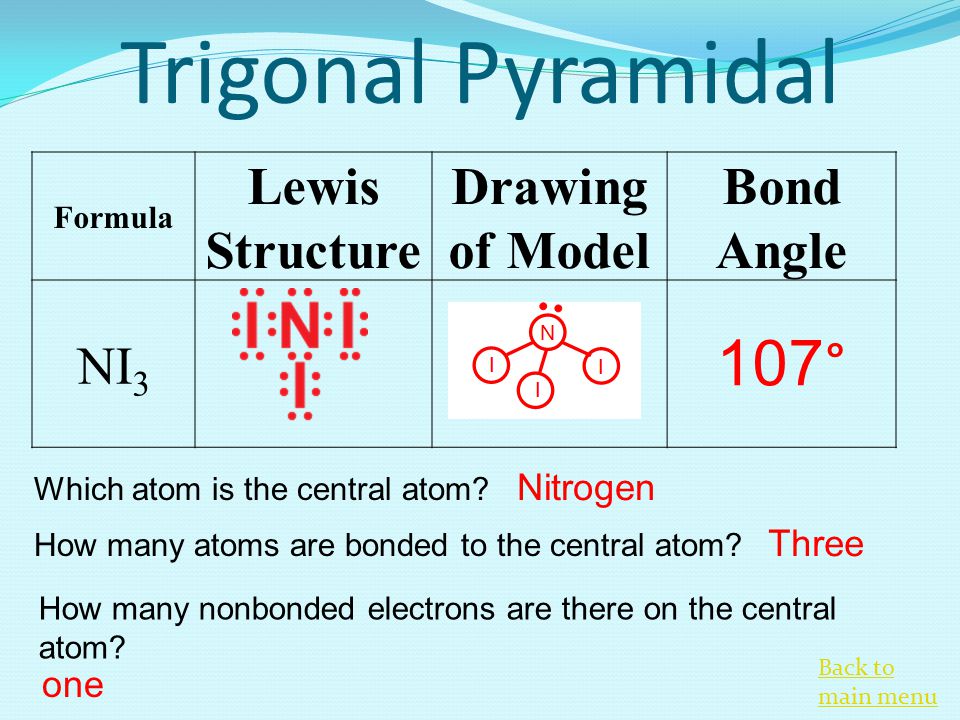
Covalent Bonding And Nomenclature Ppt Download

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Draw The Lewis Structure For Each Species Below Then Chegg Com

Ch4o Lewis Structure How To Draw The Lewis Structure For Ch4o Youtube
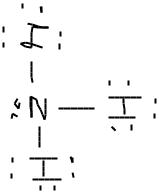
I Believe The Lewis Structure Looks Like This For Chegg Com

Oneclass What Is The Lewis Structure Of Ni3

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

What Is The Lewis Structure Of Ni3 Study Com
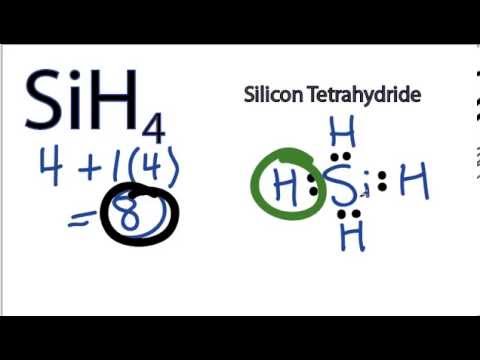
Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube
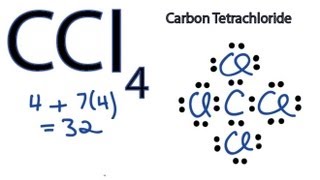
Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube

How To Draw Lewis Structure Of Ni3 Drawing Easy

