Lewis Structure Of Hno2
What is the lewis structure of HNO2. Draw the Lewis electron dot structures for these molecules.

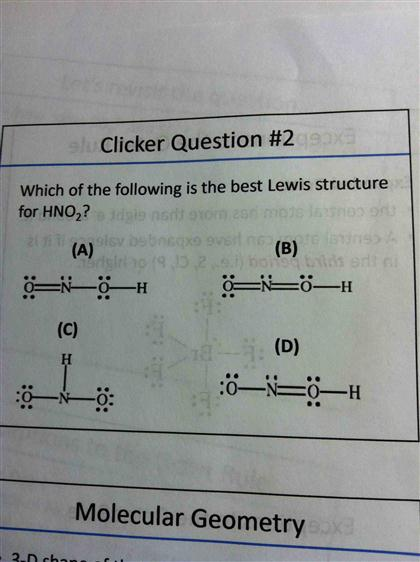
Oneclass Below Are Two Different Lewis Structures For Nitrous Acid Hno2 Which Is The Better Lewis
A step-by-step explanation of how to draw the HONO Lewis Dot StructureFor the HONO Lewis structure calculate the total number of valence electrons for the.
Lewis structure of hno2. For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule. There are one CO bond one C-O bond and one O-H in HNO 2 lewis structure. It is a conjugate acid of a nitrite.
Get the detailed answer. The actual connectivity is H-O-N-O but you could also draw a reasonable Lewis structure for H-NO2 with formal charges on N and O After that you follow the normal procedure for drawing Lewis structures. The HNO 2 Lewis structure is easier to think of if you consider it NO 2 with an H bonded to one of the oxygen atoms.
Count up the total number of valence electrons and distribute them in such a way that every atom has an octet - remembering that 2 is an octet for hydrogen. It is a conjugate base of a hydroxylamine. H3COCH3 the atoms are in the.
Nitrogen atom is the center atom in HNO 2. Predict the electron pair geometry and the molecular structure of each of the followingd ClSO S is the central atom See all problems in Electron Geometry. In HNO 2 Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
HNO2 HONOb What are the electronas medium difficulty. B Write the symbol of chlorine and represent its valence electrons with the help of crosses that is. What is the lewis structure of HNO 2.
Please do the followings for these molecules. Drawing the Lewis Structure for HNO 3. Hno2 lewis structure Resonance is a remnant of valence bond theory which is necessary because it is impossible to describe delocalised bonding within a localised bonding scheme.
It is on the World Health Organizations List of Essential Medicines a list of the most important medications needed in a. Lewis structure of nitric acid. Free unlimited access for 30 days limited time only.
However the answer then assumes that H N O X 2 has no resonance but rather one single and one double bond. A Thus an electron pair is shared between C and C lThis is the Lewis electron dot diagram for C C l 4. It tells us about bond nature molecular geometry and hybridization among other properties.
From here we can already infer that HNO2 is a polar molecule. There is a NO bond in nitric acid lewis structure. This is a pattern seen with many acids.
Draw the Lewis structure for HNO2 from the skeletals Wis structure for HNO2 from the skeletal structure presented below. Lewis structure of nitrous acid. From the Lewis structure it is clear that HNO2 is not a symmetrical molecule.
Log in Sign up. HNO2 Lewis Structure. The N O bonds in the N O X 2 X ion are equal in length whereas they are unequal in H N O X 2.
1- Name the. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. Check the formal charges to be sure that each atom has a.
One side of N is bonded to OH and the other side is a double bond with O. Since the nitrogen dioxide ion has resonance the N O bonds are equal as resonance is in reality a hybrid of all of the possible structures for a certain molecule. HNO2 the atoms are in the order HONO d.
The Lewis structure of a compound represents a graphic arrangement of constituent atoms present in a molecule mixture. Draw the lewis structure for the following. There are no lone pairs on nitrogen atom and also.
Arrange them in a sequence. Nitrous acid as sodium nitrite is used as part of an intravenous mixture with sodium thiosulfate to treat cyanide poisoning. Resonance structures are not real.
If the valence his are filled to the usual limit maximum of 8 how many non-bonding valence electrons are in the molecule. Heshan Nipuna last update. Aminooxidanide is a nitrogen oxoanion.
There are no charges on atoms and one double bond exists between nitrogen and one oxygen atom in the lewis structure of nitrous acid. Nitrous acid is a nitrogen oxoacid. Drawing the Lewis Structure for HNO 3.
One Lewis structure describes one electronic configuration with fully localised electrons. The necessary steps required to show the formation of C C l 4 by Lewis electron dot diagram has been jumbled. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms.

Nitrous Acid Structure Properties And Uses Of Hno2
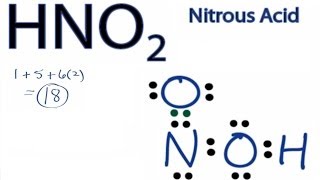
Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube

Hno2 Lewis Structure Nitrous Acid Youtube
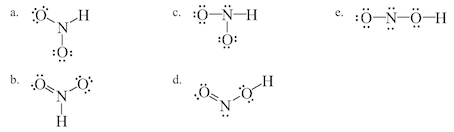
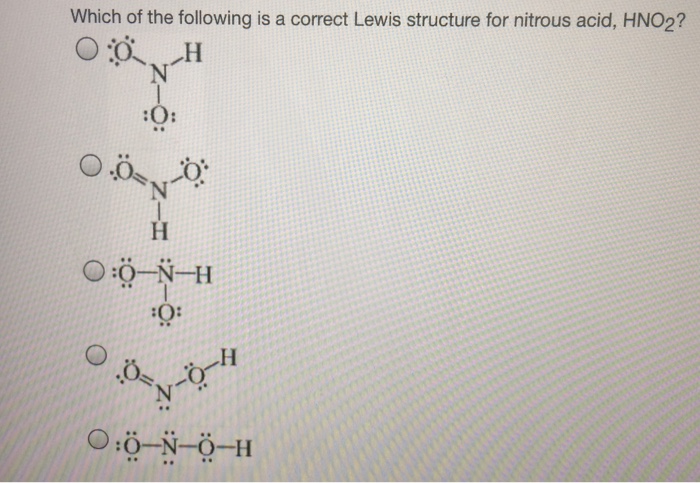
Which Of The Following Is A Correct Lewis Structure F

Hno2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Hno2 Lewis Structure Nitrous Acid Youtube
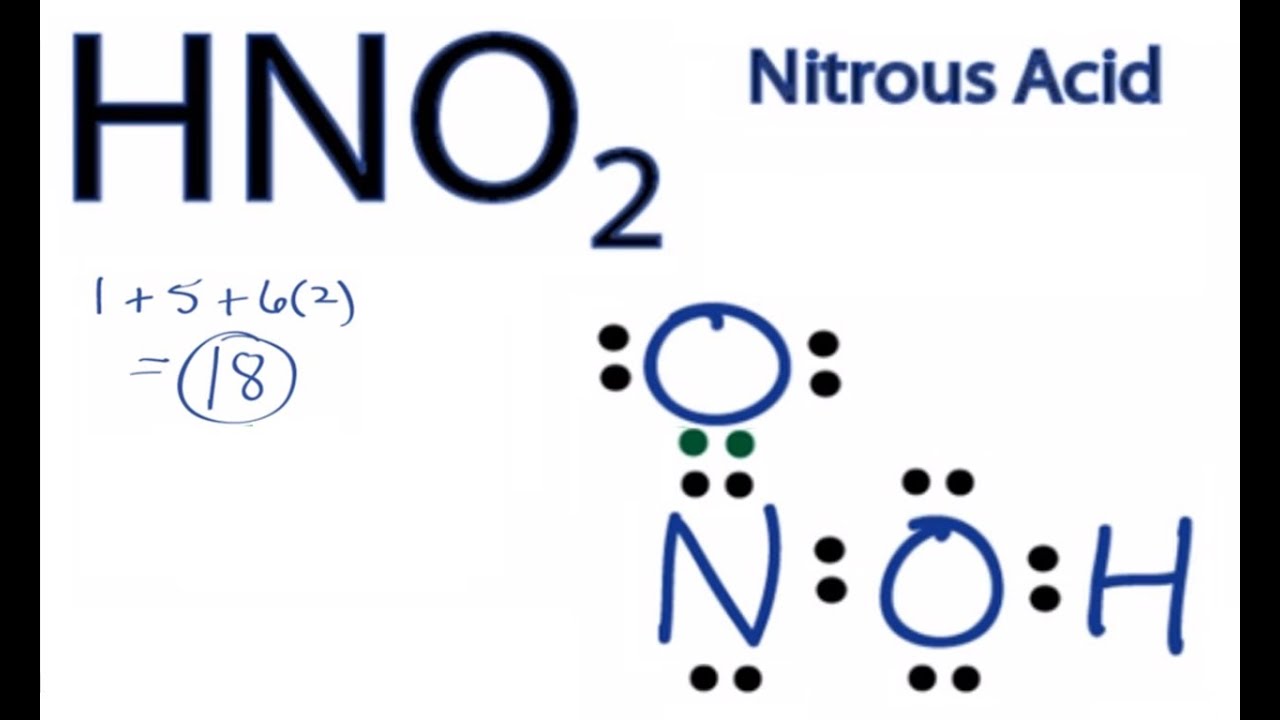
Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube

Draw The Lewis Dot Structure Of Hno2 Brainly In

Lewis Structure Stickers Redbubble

Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube

Why Does Hno2 Not Have Resonance Chemistry Stack Exchange
Solved 16 Consider Nitrous Acid Hno2 Hono A How Do I Write A Lewis Structure B What Are The Electron Pair And Molecular Geometries Of The Course Hero

Which Of The Following Is The Best Lewis Structure Chegg Com
Which Of The Following Is A Correct Lewis Structure Chegg Com
Is This A Correct Lewis Structure For Nitrous Acid How Can The Nitrogen Atom Have Two Lone Pairs Quora
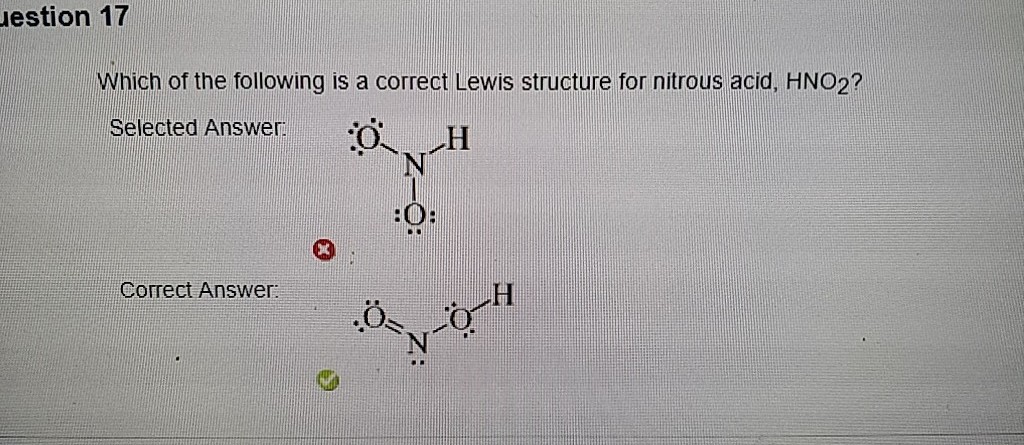
Estion 17 Which Of The Following Is A Correct Lewis Chegg Com
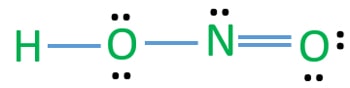
Hno2 Nitrous Acid Lewis Structure
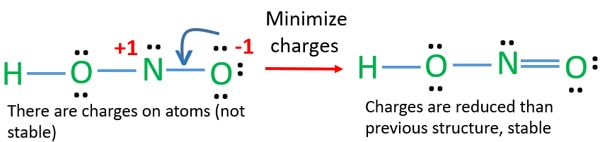
Hno2 Nitrous Acid Lewis Structure

How Would You Draw A Lewis Structure For Hno2 Quora