Lio2 Lewis Structure
The ground state gas phase Li 2 O molecule is linear with a bond length consistent with strong ionic bonding. O2- is bonded in a body-centered cubic geometry to eight equivalent Li1 atoms.
Laser-ablated lithium atoms react with oxygen molecules as do thermal lithium atoms to form the LiO2 and LiO2Li ionic molecules.

Lio2 lewis structure. The structure is three-dimensional. A step-by-step explanation of how to draw the Li2O Lewis Dot StructureFor Li2O we have an ionic compound and we need to take that into account when we draw. N 5 4 x H 4 x 1 4 -1 Total 54-1 8 electrons 4 bonds and lone pairs.
A step-by-step explanation of how to draw the AlI3 Lewis Dot StructureFor the AlI3 structure use the periodic table to find the total number of valence elec. Potassium iodide - YouTube. There are two shorter.
4 Li 2 H 2 O O 2 4 LiOH. 4 Li O 2 2 Li 2 O. NH 4 the ammonium ion.
Lewis Dot Structures can be produced by following a sequence of steps. A step-by-step explanation of how to draw the KNO3 Lewis Dot StructureFor KNO3 we have an ionic compound and we need to take that into account when we draw. Lets produce a Lewis Dot Structure for.
A step-by-step explanation of how to draw the Na2O Lewis Dot StructureFor Na2O we have an ionic compound and we need to take that into account when we draw. Solid lithium oxide adopts an antifluorite structure with four-coordinated Li centers and eight-coordinated oxides. Experimental studies indicate that the LiO 2 molecule contains highly ionic bonds.
The structure is three-dimensional. How to Draw the Lewis Dot Structure for KI. These charges came as Lithium atoms each lost one electron.
L oxyde de lithium est un composé chimique de formule Li 2 O. In addition excess energy associated with the laser ablation. Calcium nitride - YouTube.
All LiO bond lengths are 202 Å. Li2O is Fluorite structured and crystallizes in the cubic Fm-3m space group. This indicated that the force constant between the two oxygen atoms corresponds with the constant found for the O 2 ion.
Li is bonded to six equivalent O atoms to form LiO6 octahedra that share corners with eight equivalent LiO6 octahedra corners with six equivalent OLi3O tetrahedra and edges with two equivalent LiO6 octahedra. This leaves Lithium ions with a charge of 1 each and Oxygen ion with a charge of 2-. How to Draw the Lewis Dot Structure for Ca3N2.
The solid consists of layers of monovalent lithium cations Li that lie between extended anionic sheets of cobalt and oxygen atoms arranged as edge-sharing octahedra with two faces parallel to the sheet plane. VSEPR theory would predict a bent shape similar to H 2 O. Lithium acetate is an acetate salt comprising equal numbers of acetate and lithium ions.
LiO2 is Marcasite structured and crystallizes in the orthorhombic Pnnm space group. Step 2Arrange the atoms identify a central atom if possible. CID 3028194 Lithium CID 176 Acetic acid Date s.
Li1 is bonded to four equivalent O2- atoms to form a mixture of corner and edge-sharing LiO4 tetrahedra. How to Draw the Lewis Dot Structure for KI. Eighteen different values were attained using six isotopic species.
Well first draw the metal and put it in brackets. The structure of LiCoO 2 has been studied with numerous techniques including x-ray diffraction electron microscopy neutron powder diffraction and EXAFS. It is an organic lithium salt and an acetate salt.
How to draw the Lewis Structure of Oxygen Gas - with explanationCheck me out. Lithium oxide Li2O CID 166630 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. For KCl we have an ionic compound and we need to take that into account when we draw the Lewis Structure.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS. This question is best answered visually. Il se forme avec de petites quantités d hydroxyde de lithium LiOH lorsque le lithium métallique brûle dans lair et se combine avec l oxygène et l eau atmosphériques.
So here is a very helpful and detailed image I found by googling Lithium oxide Lewis Dot Structure. The corner-sharing octahedral tilt angles are 69. So basically the Lithium atoms each lose one valence electron and the Oxygen gains the 2 electrons from Lithium.
Lithium Acetate C2h3lio2 Chemspider

Draw The Lewis Structure Of Li2o Lithium Oxide Youtube

Oneclass Draw A Lewis Structure For Each Of The Following Ionic Compounds A Lif B Li2o C Sro
How To Draw The Lewis Dot Structure For Calcium Chloride Quora

Chapter 9a Chemical Bonding I Basic Concepts Ppt Download
Li2o Is Often Considered To Be Covalent In Nature Because Of The Unusually High Electro Negativity Of Lithium Sarthaks Econnect Largest Online Education Community

Nomenclature Ionic Bonding Ppt Download
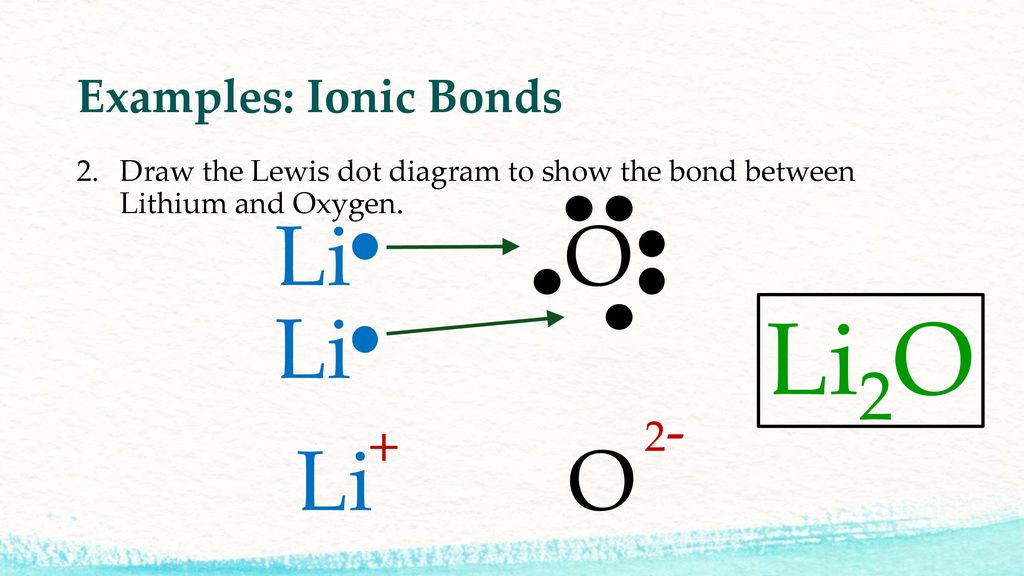
Chapter 2 The Material World Ppt Download

Nomenclature Ionic Bonding Ppt Download

Molecules And Polyatomic Ions Example 1 Video Chemistry Ck 12 Foundation

How To Draw The Lewis Dot Structure For Li2o Lithium Oxide Youtube

Unit 7 Bonding Molecular Geometry Ppt Video Online Download

Unit 7 Bonding Molecular Geometry Ppt Video Online Download

This Is How The Ionic Bond Forms In Lithium Oxide Li2o Youtube

Type Of Reaction For Li O2 Li2o Youtube

How To Draw The Lewis Dot Structure For Li2s Lithium Sulfide Youtube
What Would The Lewis Dot Structure Be For Li2o Quora

Ionic Molecular Compounds Ppt Video Online Download