(nh4)2so4 Dissolved In Water Equation
To increase the total moles of gas in the system. How many kilograms of NH3 are needed to produce 290x105kg of NH42SO4.
1 The Ionic Solid Nh4 2so4 Is A Strong Electrolyte Chegg Com
This pair of ions is the result of nh42so4 reacting with the water itself which bears the chemical formula h2o.
(nh4)2so4 dissolved in water equation. Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. When it is in the solution it forms ions. The solubility of NH42SO4 in water at 0C is 704 per 100 g of water 2 and that in anhydrous hydrogen peroxide 694 g per 100 g of H2O2 3.
Writing Dissociation Equations for Strong Electrolytes Soluble. Is NH4 2SO4 insoluble in water. 2 NH3 H2SO4 -.
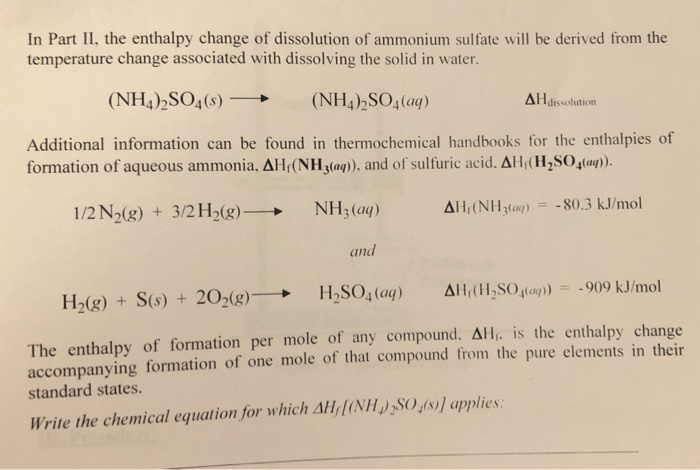
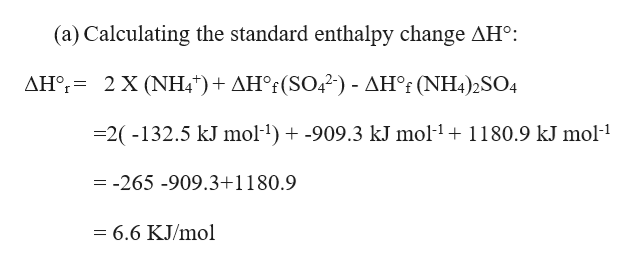
See the answer. NH42SO4 s ------2NH4 aq SO42- aq a Calculate the standard enthalpy change ΔH for this reaction using the following data. Hence let us balance that first as.
I get so lost on conversions and mols and all of that Ive asked for help 2 NH42SO4 2NH4 SO4 2- The valency of sullphate SO4 2- anion is 2 and not 1. Now let us balance the equation. When nh42so4 is dissolved in water nh4 and so42- are produced.
1915 Fx Lol TH Normal BI IU Billi Bali HClaq NHOHaq C2H5OHag NH4Cl H20---- Rewrite the chemical equation for the dissolution of NH4Cl in water to include the heat term. There are 2 ferric ions in the reactant side but only one in the product side. Chemistry 22062019 0440 deedee363 will mark you brainliest 15 points why does the equilibrium of a system shift when the pressure is increased.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and. Fe2SO4 3NH4OH -FeOH 3NH4 2SO4. NH42SO4 is an ionic compound that dissociates itself upon its addition to the solvent to form a solution.
2NH 4 VO 3 SO 2 2H 2 SO 4 2VOSO 4 NH 4 2 SO 4 2H 2 O. To maximize the stress on the system b. Answer 1 of 1.
To stop restoring equilibrium to the system c. The compound ammonium sulfate NH42SO4 is soluble in water. This problem has been solved.
NH42SO4 2NH4 SO4-2 05 mole NH42SO4 must produce 1. In this video we will describe the equation NH42SO4 H2O and write what happens when NH42SO4 is dissolved in waterWhen NH42SO4 is dissolved in H2O w. Ammonium metavanadate react with sulfur dioxide and sulfuric acid to produce vanadyl sulfate ammonium sulfate and water.
The compound ammonium sulfate is a enthalpy change of dissolution compous nh4 2so4 equation for nitrate aluminum salts and ammonia precipitation reactions preparation from iron ii hexahydrate major classes chemical. 1038 g100 g water at 100 it is widely used as a fertilizer for alkaline soils. They are known as the products of the reaction whereas nh42so4 and h20 are the reactants.
What ions are present when nh42so4 is dissolved in water. To tell if NH42SO4 Ammonium sulfate forms an acidic basic alkaline or neutral solution we can use these three simple rules along with the neutralizat. In this equation we have ammonia and water in the reactant side.
A saturated solution of ammonium sulfate NH42SO4 in water at 30 0C contains 780 g NH42SO4 per 1000 g H2O. Write the net ionic equation for the dissociation reaction that occurs when solid ammonium sulfate dissolves in water. With a chemical formula of NH43PO4 ammonium phosphate is found in crystalline powder form and is soluble in water.
3 Get Other questions on the subject. What ions are present when nh42so4 is dissolved in water. Lammoniaque désigne la solution aqueuse basique de.
Fe2SO4 3NH3H2O-FeOH 3NH4 2SO4. The balanced dissociation for for NH42SO4 is. ΔHf NH42SO4 s -11809 kJ mol-1 ΔHf NH4 aq -1325 kJ mol-1 ΔHf SO42- aq -9093 kJ mol-1 b Calculate the temperature.
Many forget that water. Mole NH4 2 x 05 1and 05 mole SO4-2. A high-melting decomposes above 280 white solid which is very soluble in water 706 g100 g water at 0.
Ions are often produced when simple molecules are dissolved in water. Chemistry 22062019 0230 chloe9869. We can consider this as ammonium hydroxide for balancing purpose.
What is the molality of this solution. The reaction could not take place if nh42so4 was not soluble in water but as it is ions are produced. Use the pull-down boxes to specify states such as aq or 8 но Submit Answer Retry.
The salt ammonium sulfate dissolves in water according to the reaction. 1 Write the equation for the dissolution of each of the following in water and then determine the number of moles of each ion produced as well as the total number of moles of ions produced. The balanced equation is.
Other questions on the subject.

Is Nh4 2so4 Acidic Basic Or Neutral Dissolved In Water Youtube

How To Write The Net Ionic Equation For Ca No3 2 Nh4 2so4 Caso4 Nh4no3 Youtube
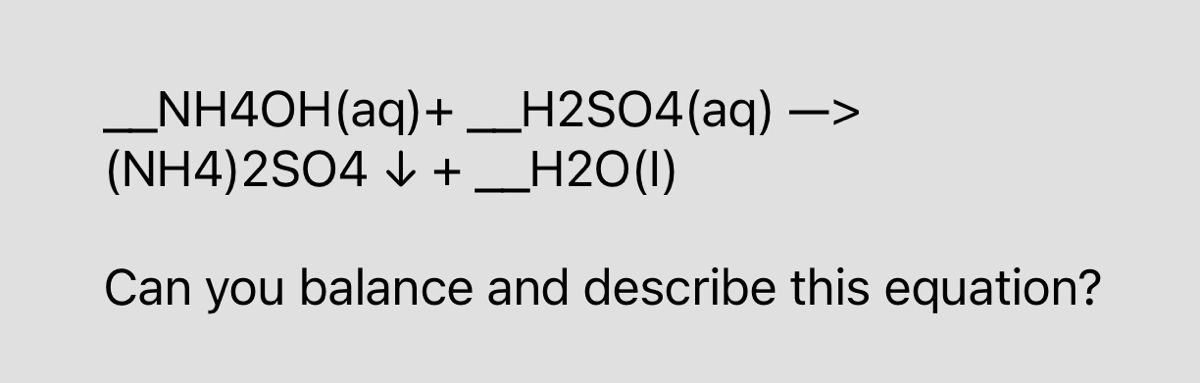
Answered Nh4oh Aq H2so4 Aq Nh4 2so4 V Bartleby

The Compous Ammonium Sulfate Nh4 2so4 Is Soluble In Chegg Com

Solubility Of Qr2 Versus Nh4 2so4 At Ph 8 20mm Tris Hcl And 150mm Download Scientific Diagram
How To Write The Net Ionic Equation For Nh4 2so4 Koh K2so4 Nh3 H2o Youtube
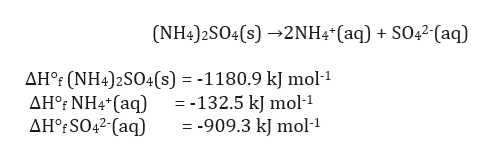
Answered The Salt Ammonium Sulfate Dissolves In Bartleby
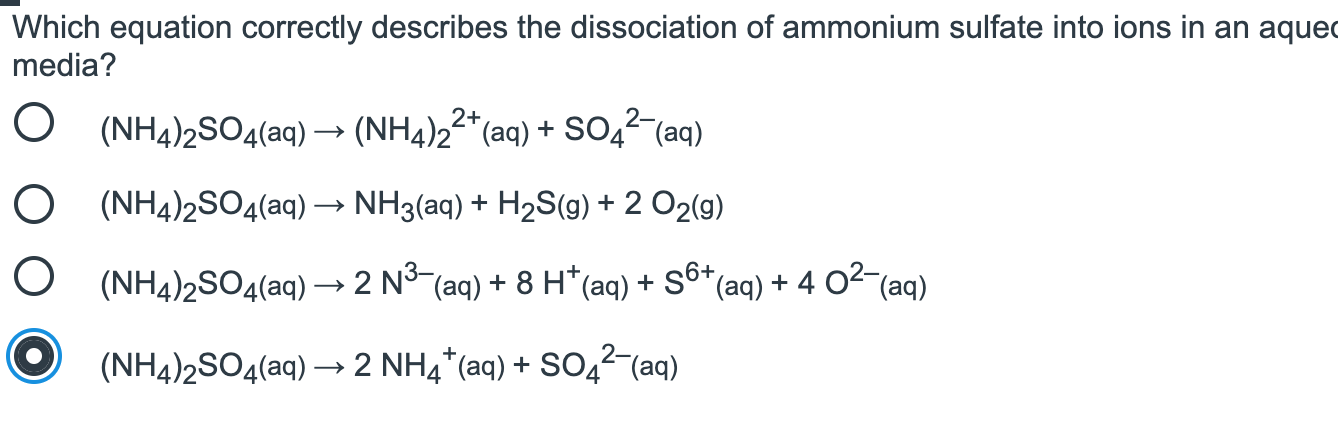
Which Equation Correctly Describes The Dissociation Chegg Com
How To Balance Nh4 2so4 Ba No3 2 Nh4no3 Baso4 Youtube
Types Of Aqueous Reactions Ppt Video Online Download

How To Balance H2so4 Nh4oh Nh4 2so4 H2o Sulfuric Acid Ammonium Hydroxide Youtube

Answered The Salt Ammonium Sulfate Dissolves In Bartleby

Answered The Salt Ammonium Sulfate Dissolves In Bartleby

Equation For Nh4 2so4 H2o Ammonium Sulfate Water Youtube

Is Nh4 2so4 Soluble Or Insoluble In Water Youtube
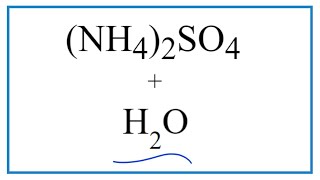
Equation For Nh4 2so4 H2o Ammonium Sulfate Water Youtube
In Part Ii The Enthalpy Change Of Dissolution Of Chegg Com

How To Write The Net Ionic Equation For Nh4 2so4 Naoh Na2so4 Nh3 H2o Youtube

Types Of Aqueous Reactions Ppt Video Online Download