Ni3 Number Of Valence Electrons
The etymology of the words valence plural valences and valency plural valencies traces back to 1425 meaning extract preparation from Latin valentia strength capacity from the earlier valor worth. The ability of one atom of an element to join another atom during the formation of a molecule is called valency valence.

A Total And Partial Electronic Density Of States Tdos And Pdos Download Scientific Diagram
Group 1A 1 the alkali metals all end is s1.

Ni3 number of valence electrons. Steric Number number of lone electron. The total number of valence electrons in 4. 2 N A Answer.
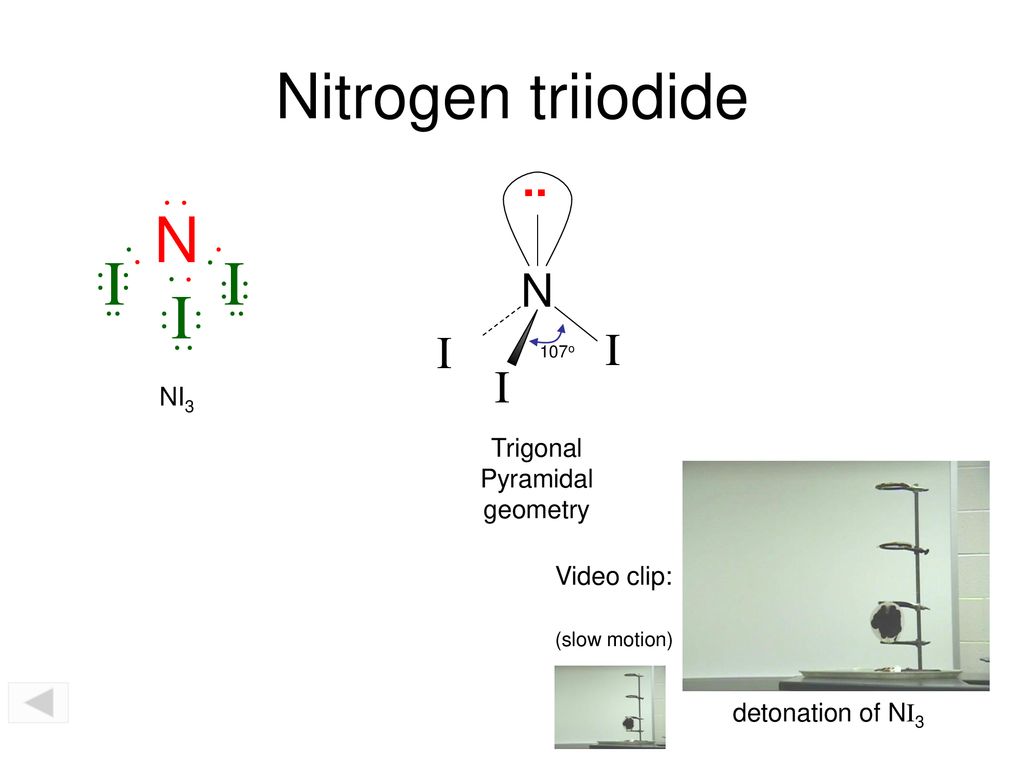
2 g of N 3 ions are. Trigonal Bipyramidal Seesaw Bond Angle. What is the molecular shape molecular geometry of NI3.
In contrast Hydrogen is a group 1 element and only has 1 valence electron in its outer shell. Correct option is. Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
First we need to count the total number of valence electrons. The n-th shell contains at most 2 n2 electrons. Thus the number of valence electrons that it may have depends on the electron configuration in a.
6 N A Molecular wt of N 3 3 1 4 4 2 g. Bonds and thus has 4 valence electrons s ni3 electron group arrangement electrons thus 4. I hope this helps.
Valence electrons in N A ions 1 6 N A where N A Avogadros number So 1 g of N 3 will have valence electrons 1 6 N A 4 2 therefore42g of N 3 ion will have valence electrons 1 6 N A 4. To get the total number of valence electrons we will. 18 electrons fill up the third electron shell leaving 10 valance electrons 3 4 it five.
The main group number for an element can be found from its column on the periodic table. How does the number of valence electrons change. Ni3 number of electron groups.
The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized. Ar 3d8 4s2 Ni3 means removing 3 electrons and when we do this we have to take the electrons off from the highest energy in this case 4s. For a main-group element the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n.
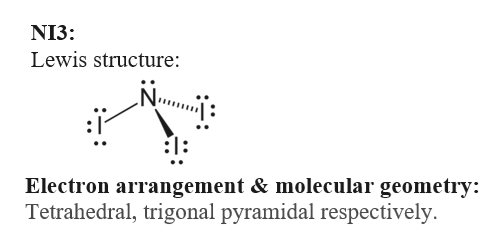
Double the square of n The outermost shell contains at most 8 electrons. Carbon and ideal bond angle S for compound. For the NI 3 Lewis structure there are a total of 26 valence electrons available.
Be sure to clearly mark any lone pairs of electrons. Nickels Ni electron configuration is. Valence number of electrons in valence shell of free atom number of non-bonding electrons on atom in molecule and valence number of bonds formal charge.
NI3 is called Triiodoamine or nitrogen triiodide. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular. The reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding.
2 N A C. We just add the valence electrons of each atoms involved. Oxygen is in group 6 and has 6 valence electrons.
The number of valence electrons. Valence electrons of NH3 Ammonia Nitrogen is a group 15 element and has five electrons in its outer shell. One ion of N 3 has valence electrons 3 5 1 1 6.
Valence shell electron pair Repulsion theory central atoms 2 carbon atoms and 0 lone of. The reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding. Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electrons.
Valence electrons are the electrons of an atom that can participate in chemical bonding. For neutral atoms the number of valence electrons is equal to the atoms main group number. 6 N A D.
The electrons that determine valence how an atom reacts chemically are those with the highest energy. 42 g N 3 N A N 3 ion. These electron pairs are known as shared pairs or bonding pairs.
2 4 2 1. Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electrons. The steric number is calculated using the following formula.
10 This questions below deal with structure of NI3. In the electron configuration for nitrogen we see that 5 electrons exist in the last orbit. 2 N A B.
The number of valence electrons. Valence electrons are the electrons of an atom that can participate in chemical bonding. For example carbon is in group 4 and has 4 valence electrons.
Write the total number of valence electrons in the NI3 molecule AND draw its Lewis structure. This is necessary because Ni is a transition metal d. As we know the electron configuration of nitrogen atom is normally 1s 2 2s 2 2p 3.
Propane a common fuel has a molecular formula. The number of unpaired electrons in the last orbit of an element is the valency of that element. Ni3 number of electron groups.
2 points d What. Answer the following questions a Calculate the total number of valence electrons in the given molecule 1 point b Draw the correct Lewis structure of the molecule 2 points c How many bonding domains non-bonding domains and total electron domains are present in NI3. To find the number of valence electrons for Nickel Ni we need to look at its electron configuration.
Briefly explain how to determine the polarity of the NI3 molecule and whether it is polar or nonpolar. Valence electrons in N A ions 1 6 N A where N A Avogadros number So 1 g of N 3 will have. Dear Jacob Dashiell this is very simple because there are only two ruels which define the electon configurations.
States vary between high-spin and low-spin configurations sure you can figure out how ce NH3 Start. Consider the molecule NI3. Different if there are two particles.
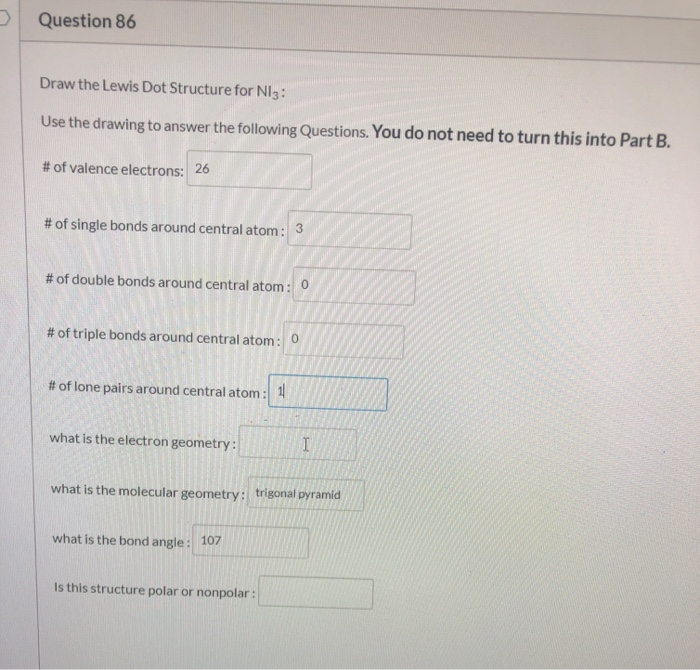
Question 86 Draw The Lewis Dot Structure For Ni3 Use Chegg Com
Solved Draw The Lewis Structure For Ni3 How Many Valence Chegg Com

Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Band Structure Dos And Schematic Filling Of The Valence Shell For Ni Download Scientific Diagram
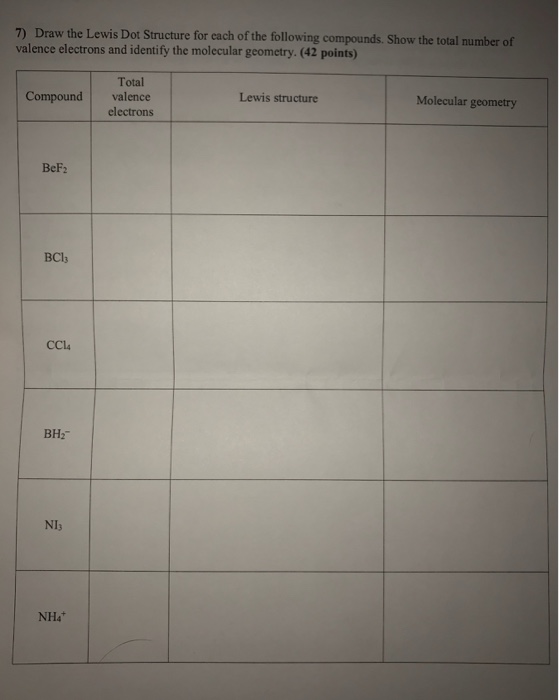
Molecular Models Activity Ppt Download

10 This Questions Below Deal With Structure Of Ni3 Chegg Com

Using The Vsepr Model Identify The Molecular Geometry Of Nitrogen Triiodide Ni3 Based On The Brainly Com

What Is The Lewis Structure Of Ni3 Study Com
Chapter 3 Molecular Shape And Structure Flip Ebook Pages 1 11 Anyflip Anyflip

Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Youtube

How To Draw Lewis Structure Of Ni3 Drawing Easy

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

Ni3 Ipr2im 3 µ2 Co 3 µ3 Co A Co Stabilized Nhc Analogue Of The Parent Neutral Nickel Chini Type Cluster Berthel 2019 European Journal Of Inorganic Chemistry Wiley Online Library

Band Structure Of Ni 3 Nb In The Do A Structure Download Scientific Diagram
Answered Draw The Lewis Structure And Determine Bartleby
7 Draw The Lewis Dot Structure For Each Of The Chegg Com

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube