Pcl3 Electron Geometry And Molecular Geometry
According to VSEPR electron pairs distribute themselves around a central atom in such a way as to maximize their distance from each other. Hydrogen iodine and carbon come from the1st 17th and 14th family groups in the periodic table.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Drawing and predicting the CH3Br molecular geometry is very easy by following the given method.
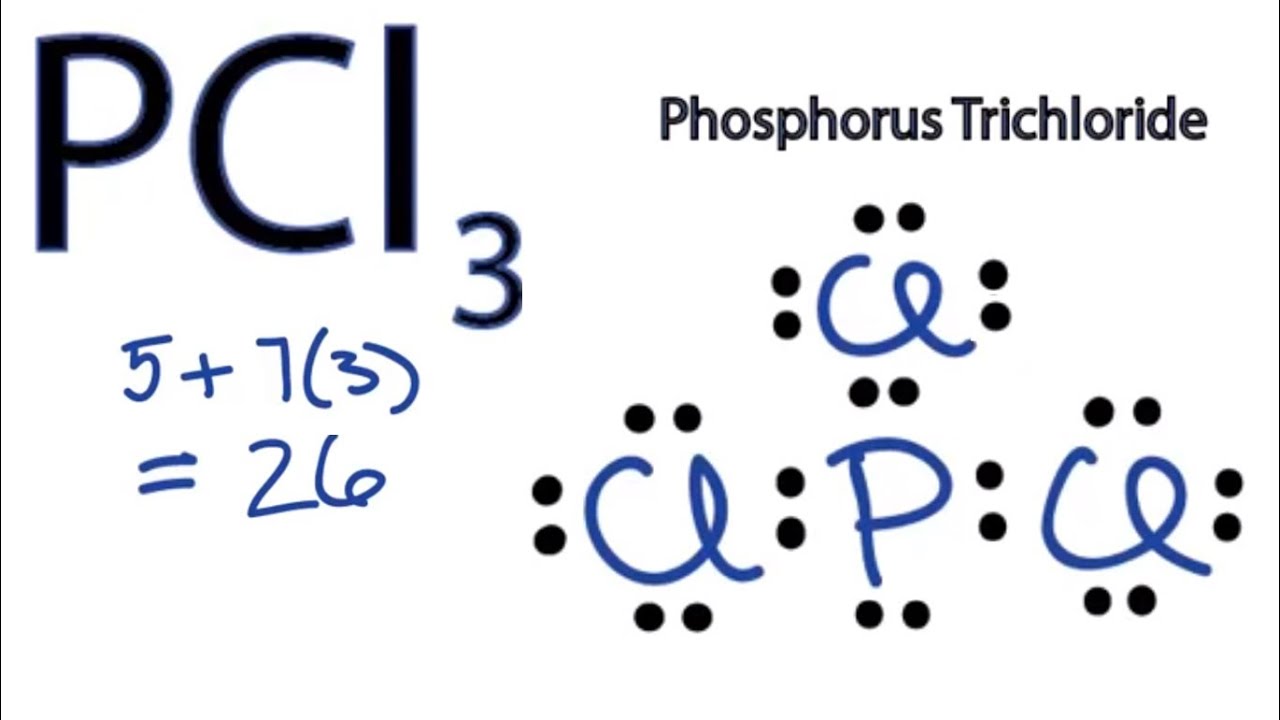
Pcl3 electron geometry and molecular geometry. There are a total of 26 valence electrons for PBr3. In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom. Rotate the molecule to observe the complete geometry.
Molecular geometry is an extension of the 2-dimensional diagram as in the below image. This is due to PCl3 being sp3 hybridized. A 1trigonal planar 2tetrahedral 3trigonal pyramidal b where x represents the outer atoms in each molecule.
It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. If the molecule has atoms on all these ends molecular shape around the central atom will be just the same as determined by electron geometry. Hydrogen bromine and carbon come from the1st 17th and 14th family groups in the periodic table.
Click to see full answer. It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. Hydrogen iodine and carbon have one seven and four valence electrons respectively.
Here in this post we described step by step to construct CH3Br molecular geometry. What is the shape of PCl3. Electron domain geometries are based on the total number of electron pairs while molecular geometries describe the arrangement of atoms and bonding pairs in a molecule.
If the molecule has atoms on all these ends molecular shape around the central atom will be just the same as determined by electron geometry. The shape of a PCl3 molecule is Trigonal pyramidal. Drawing and predicting the CH3I molecular geometry is very easy by following the given method.
This is due to PCl3 being sp3 hybridized. The molecular geometry in addition to being a 3-dimensional representation of the data at our disposal is also essential to observe and subsequently infer the reason behind the specific properties a compound exhibits. Determine the polarity of molecules.
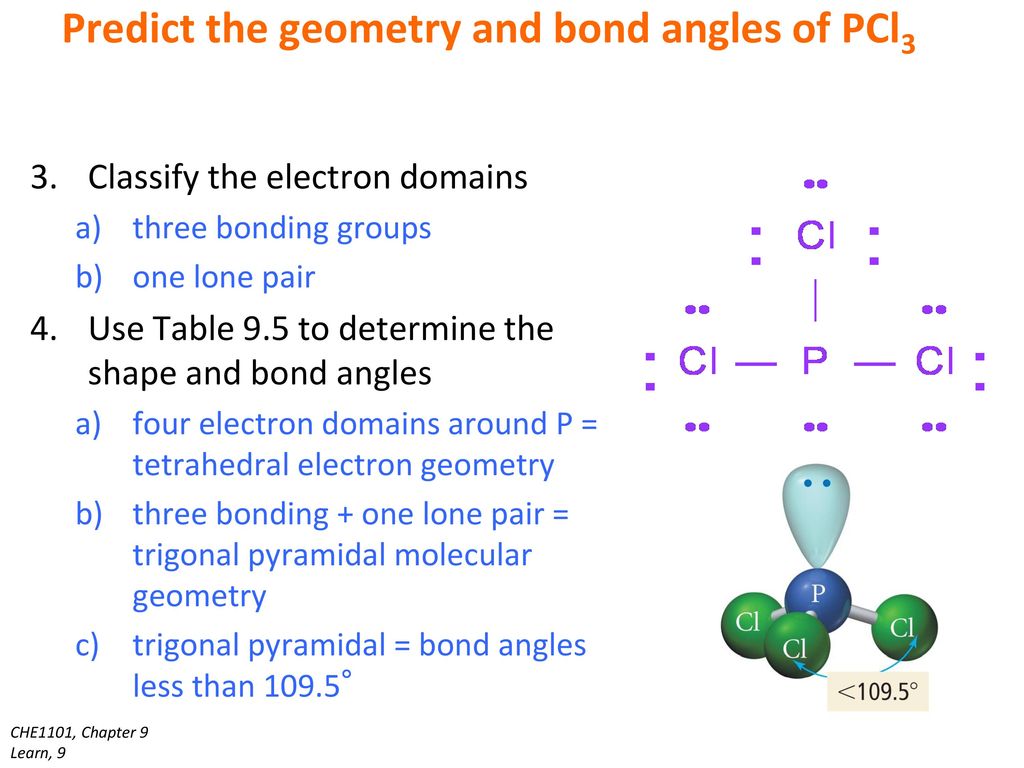
It has sp3 Hybridization and the bond angle is approximately 1095. Name the electron group geometry and molecular structure and predict the bond angle. The central P atom has one lone pair of electrons and three bond pairs of electrons.
Then add one single bond and one lone pair. Why is PCl3 tetrahedral. Hydrogen bromine and carbon have one seven and four valence electrons respectively.
Starting with the central atom click on the double bond to add one double bond. Use the Molecule Shape simulator to build a molecule. The molecule is trigonal pyramidal-shaped and is a polar molecule.
Pcl3 bond angles and shape. According to the VSEPR theory the molecular geometry of NCl3 is trigonal pyramidal and electron geometry is tetrahedral because nitrogen being pentavalent has Sp³ hybridization with 5 valence electrons in its outermost shell and it makes three bond pairs one with each chlorine atom. A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond anglesLooking at the PCl3 Lewis structure we can see that the.
This is a video worked example for the Lewis structure and moleculargeometry of phosphorus trichloride PCl3. A quick explanation of the molecular geometry of pcl3 including a description of the pcl3 bond based on vsepr theory valence shell electron pair repulsion theory the electron clouds on atoms and lone pair of electrons polar molecules tutorial. Molecular Geometry of PCl5.
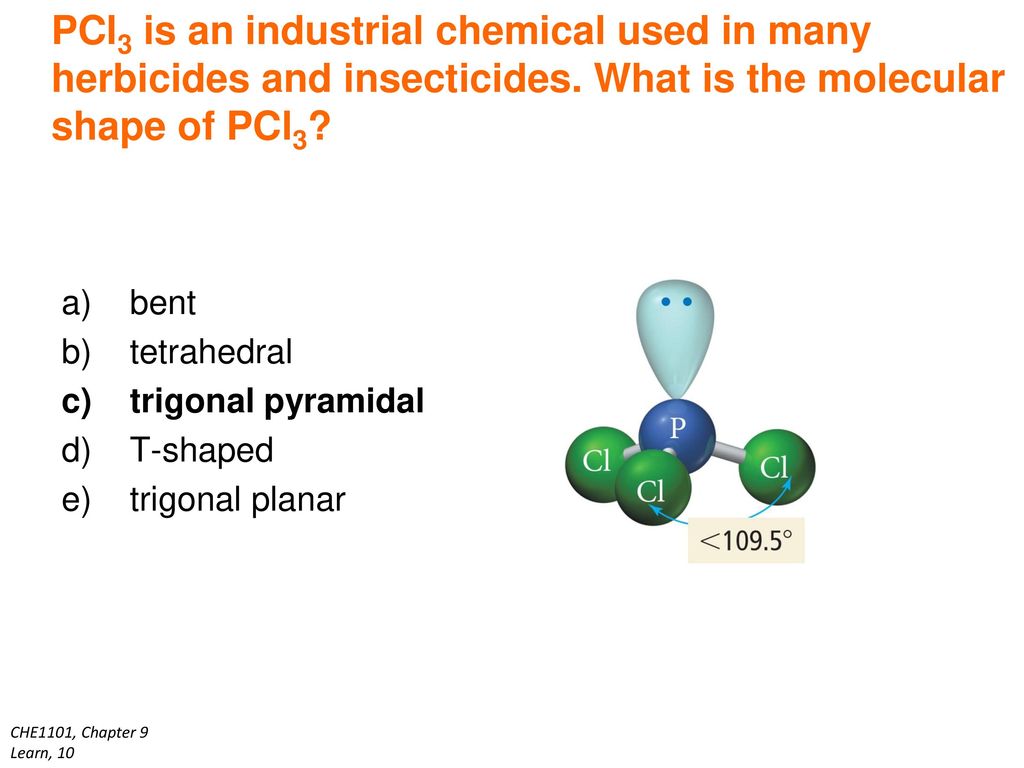
PCl3 Shape Phosphorus Trichloride has a trigonal pyramidal shape as the electrons are arranged in a tetrahedral geometry. The shape of a PCl3 molecule is Trigonal pyramidal. The central P atom has one lone pair of electrons and three bond pairs of electrons.
By Jan 7 2021 Uncategorized 0 comments Jan 7 2021 Uncategorized 0 comments. Here in this post we described step by step to construct CH3I molecular geometry.

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
What Is The Bond Angle Of Pcl3 Quora

How Many Lone Pair Electrons Are There In Pcl3 Quora
What Is The Molecular Shape Of Pcl3 Quora
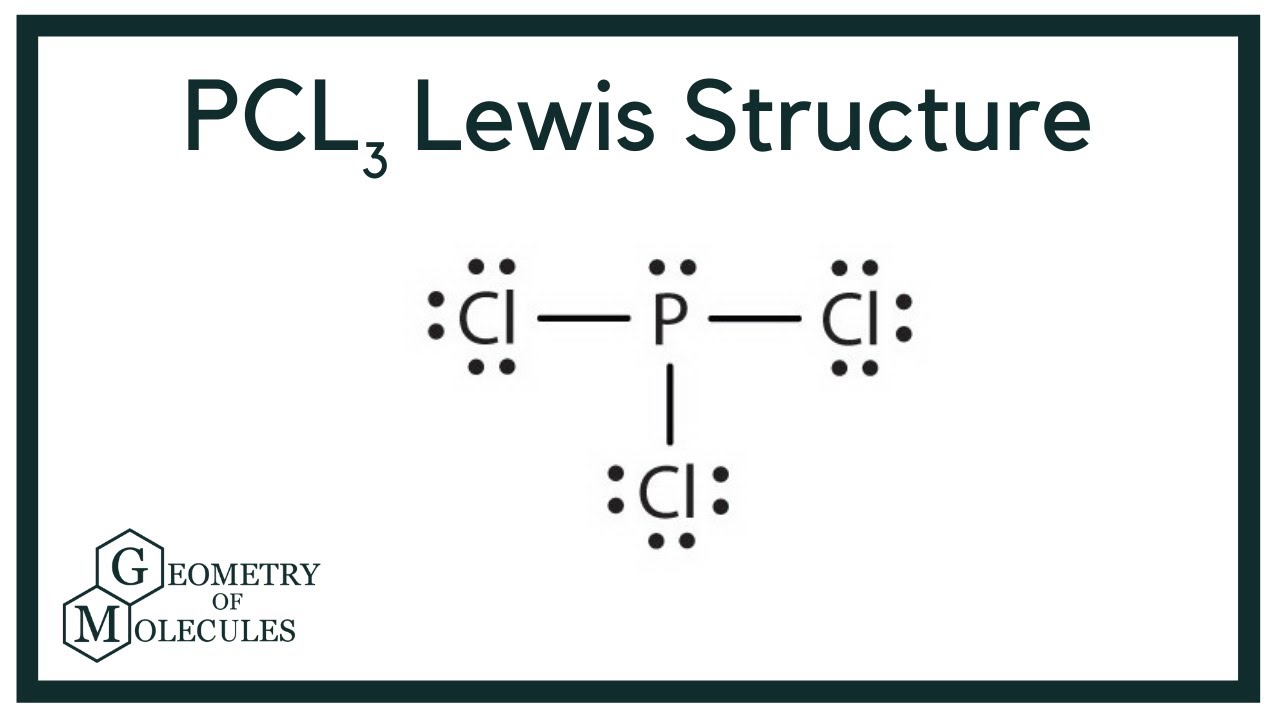
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
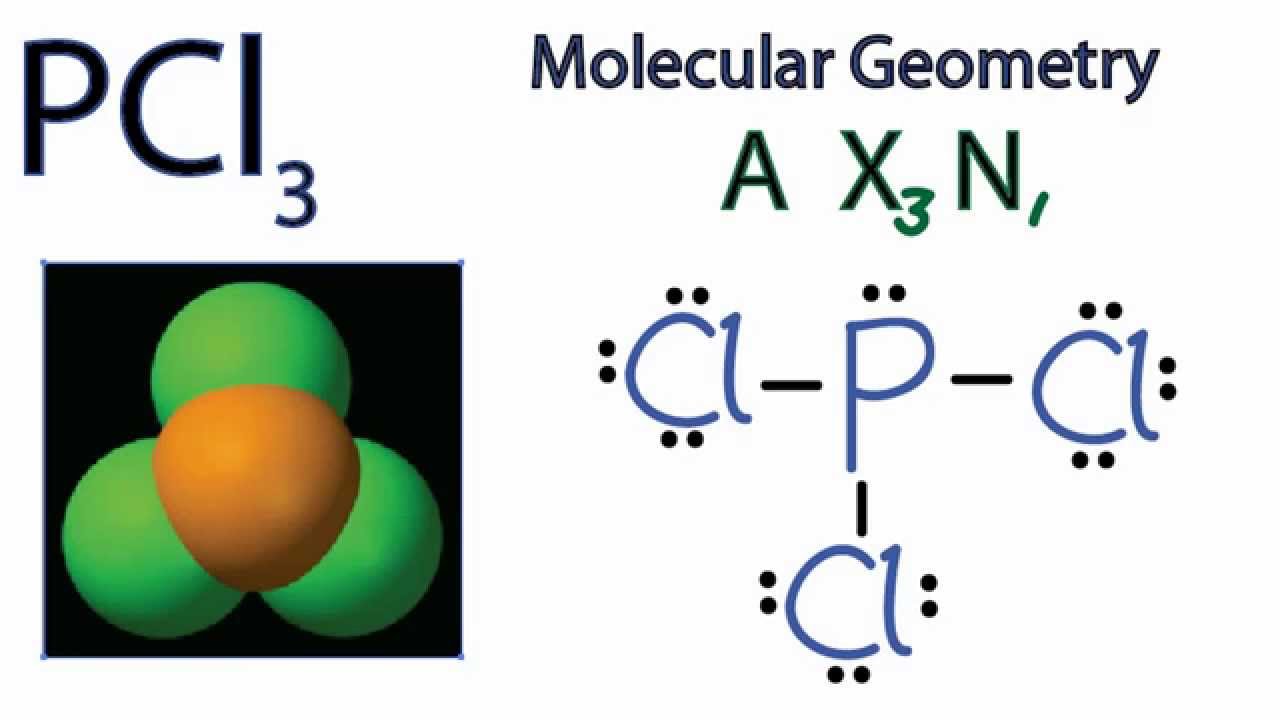
Pcl3 Molecular Geometry Shape And Bond Angles Youtube

What Is The Molecular Shape Of Pcl3 Quora

What Is The Molecular Shape Of Pcl3 Quora

How Can The Molecular Geometry Of Phosphorus Trichloride Be Described Quora

Pcl3 Lewis Structure And Molecular Geometry Youtube
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have
Https Www Mctcteach Org Chemistry C1020 C1020 Handouts Molecular 20modeling 20v 8 18 Pdf

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

What Is The Molecular Geometry Of Pcl3 Study Com

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download