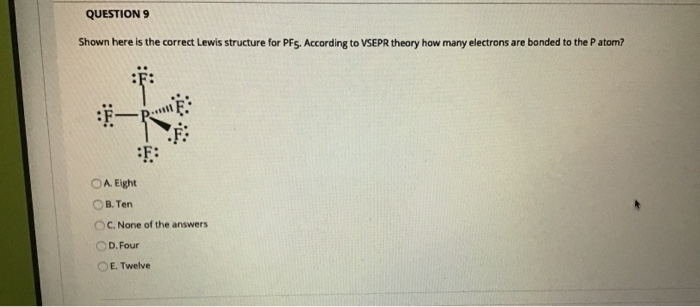
Shown Here Is The Correct Lewis Structure For Pf5
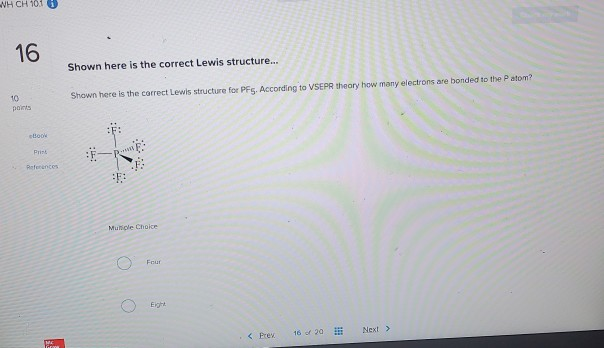
Shown here is the correct Lewis structure for PF5. According to VSEPR theory how many electrons are bonded to the P atom.
Shown Here Is The Correct Lewis Structure Shown Chegg Com
According to VSEPR theory how many electrons are bonded to the P atom in PF5.
Shown here is the correct lewis structure for pf5. According to VSEPR theory how many electrons are bonded to the P atom in PF5 Four Eight Ten Twelve None of the above. According to VSEPR theory how many electrons are bonded to the P atom. Shown here is the correct Lewis structure for PF5.
Then draw the 3D molecular structure using VSEPR rules. Shown here is the correct Lewis structure. Draw the Lewis structure for the CCl4 Select the correct hybridization for the central atom based on the electron geometry CCl4 Draw the Lewis structure for the NH3.
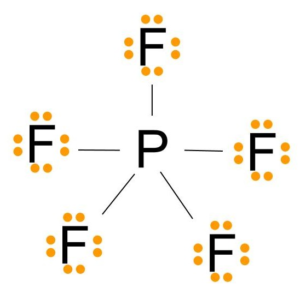
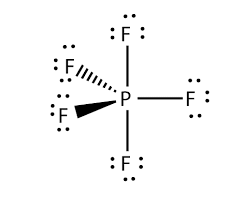
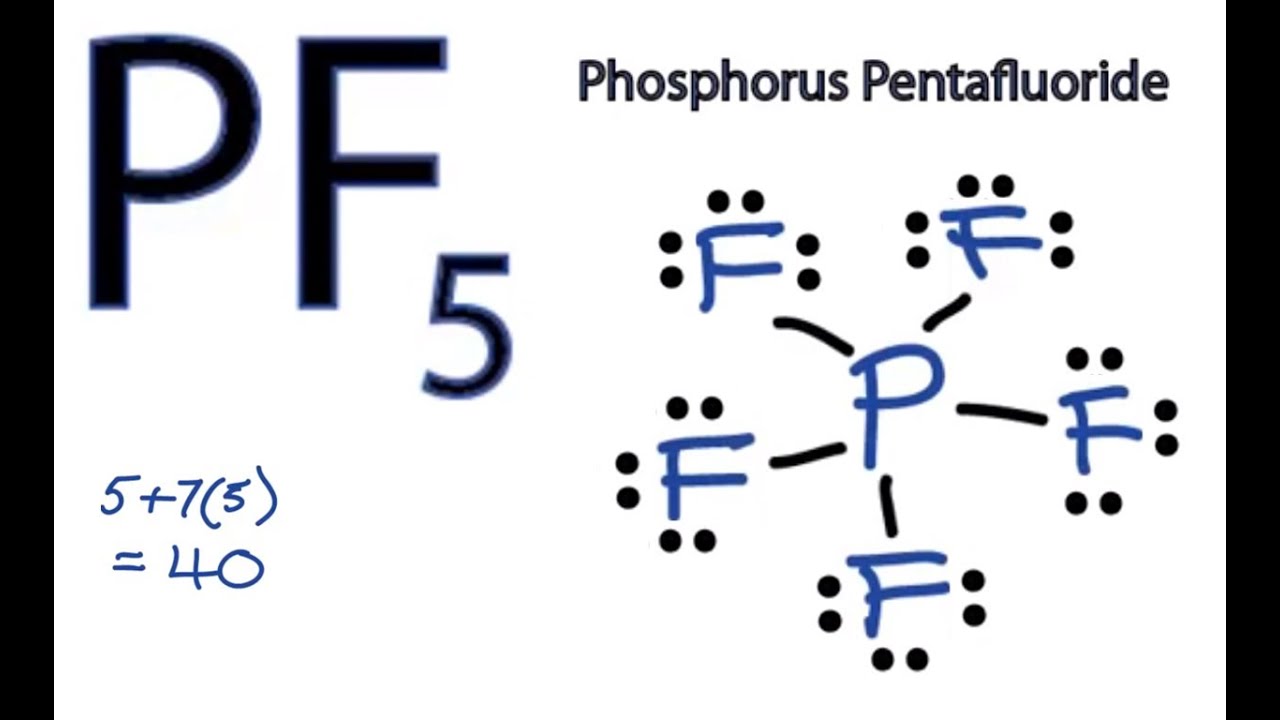
A hypervalent molecule the phenomenon is sometimes colloquially known as expanded octet is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. PF5 - Phosphorus Pentafluoride. Fluorine group 7 but we have five of those so we need to multiply that 7 by 5.
Iodine is below Period Two on the periodic table so it can have an expanded octet hold more than eight valence electrons. Asked Aug 16 2019 in Chemistry by mirandahuang. Lets do the Lewis structure for PF5.
The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. Well put the Phosphorus in the center and then the Fluorines we have five of them lets put them around it like this. Phosphorus pentafluoride was first prepared in 1876 through fluorination of phosphorus pentachloride using arsenic trifluorideOther routes to PF5 have included fluorination of PCl5 by HF AgF benzoyl fluoride SbF3 PbF2 or CaF2It can also be made by the reaction of PF3 and fluorine chlorine or chlorine in contact with calcium fluoride.
Shown here is the correct Lewis structure. Shown here is the correct Lewis structure for PF5. How does the geometrical structure of PF 5 differ from that of IF 5.
B There Are Too Many Bonding Electrons Shown. A B C 7 What Is Wrong With The Lewis Structure Shown For Sulfur Trioxide SO3. Asked Aug 16 2019 in Chemistry by mirandahuang.
The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. On the periodic table Phosphorus is in group 5 it has 5 valence electrons. Show all valence electrons in your structures.
Select the correct hybridization for the central atom based on the electron geometry NH3. According to VSEPR theory how many electrons are bonded to the P atom. According to VSEPR theory how many elect bonded to the Pato Muncle Choice O Four Prev 16 of 90 Next Question.
Draw a Lewis structure for PF 5 that shows the correct atom arrangement predicted by VSEPR theory. Note that the sum of the formal charges in each case is equal to the charge of the ion 1. 90 and 120 39.
See the answer See the answer See the answer done loading. This problem has been solved. First draw the Lewis dot structure.
PF 5 is trigonal bipyramidal whereas IF 5. Phosphorus pentachloride PCl 5 sulfur hexafluoride SF 6 chlorine trifluoride ClF 3 the chlorite ClO 2 ion and the triiodide I. Draw the Lewis structure for the OF2.
A Oxygen Not Sulfur Should Be The Central Atom. By the reaction of FSO3H on fluoride and phosphate. Lewis and Three-Dimensional Structures Trigonal Bipyramid 3-Dimensional View of PF 5 Chemistry Home Dr.
B On the basis of the Lewis structures drawn in part a answer the following questions about the. One point is earned for each correct structure. Drawing the Lewis Structure for IF 5.
What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here. In benzene C6H6 what is the hybridization of each carbon atom.
A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot. List all of the bond angles present in a molecule with a trigonal bipyramidal geometry. Sp sp2 sp3 sp3d sp3d2.
In the Lewis structure for IF5 youll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure. Shown here is the correct Lewis structure for PF5. Drawing the Lewis Structure for IF 5.
Shown here is the correct Lewis structure for PF5. A Draw the Lewis structure electron-dot diagram of each of the four species. Sundin Home sundinuwplattedu.
Therefore this molecule is nonpolar. C The Structure Shows 26 Valence Electrons But There Should Only Be 24 D Sulfur Should Have 10 Electrons Around.

Chemical Bonding I Basic Concepts Chapter 9 Copyright
Is It True That Pf5 Is A Polar Molecule Quora
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
Draw Lewis Structures For H2co3 Sf6 Pf5 If7 And Cs2 Is The Octet Rule Obeyed In These Cases Sarthaks Econnect Largest Online Education Community
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Chapter 2 Molecular Structure And Bonding

Pf5 Molecular Geometry Shape And Bond Angles Youtube
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity
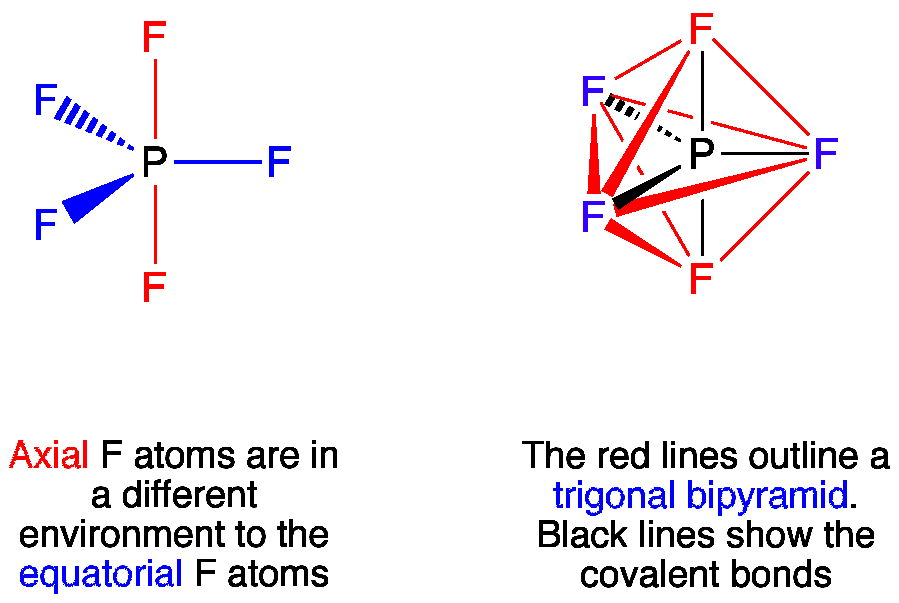
Vsepr Pf5 Phosphorus Pentafluoride

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Which Of The Following Elements Behaves Chemically Chegg Com
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
Solutions Chemistry Libretexts
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora

Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube