Consider The Lewis Dot Structure For Water Shown Here
A step-by-step explanation of how to draw the HClO4 Lewis Structure Perchloric AcidWhen we have an H or H2 in front of a polyatomic molecule like CO3. Water has also a high surface tension and adhesion.

Makethebrainhappy The Lewis Dot Structure For H2o
Click hereto get an answer to your question Consider the above Lewis Dot structureThe formal charges on Cl and O are respectively.

Consider the lewis dot structure for water shown here. Chemists usually indicate a bonding pair by a single line as shown here. In SO2 Lewis structurethere are two double bonds that are going from the atom sulphur to oxygens. In b we allow single electrons - one each from hydrogen and oxygen - to form a bonding pair between the nuclei.
In SO2 Lewis structure. All this is caused by the simple structure of h2o represented by the lewis dot diagram above. Right click HERE to open link in new window or tab.
Which of the following would be an important part of a multi-step plan to restore the stability of the red-cockaded woodpeckers ecosystem. With two bonding pairs and two lone pairs the oxygen atom has now completed its octet. When hydrogen forms a bond with oxygen it can NOT fit any more electrons around it since it only has the 1st energy level.
A lewis symbol is a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. It has totally 6 electrons in the outer shell and therefore needs two more electrons to complete octet. Consider the lewis dot structure shown here.
Consider the lewis dot structure shown here Answers. We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols. The eight total valence electrons are explicitly represented.
Alternate view of lewis dot structure of water. If link does not work draw the Lewis-dot structure to answer the questions. In c we have replaced both bonding pairs with a line or dash to symbolize the covalent bond formed.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure AmmoniaFor the NH3 structure use the periodic table to find the total number of vale. Biology 21062019 2330 BlueLemonWater. The atomic number of oxygen is 8 with electronic configuration as 1s 2 2s 2 2p 4.
Lewis structure for water Water has the molecular formula H2O. Lewis Dot Structure of H2O Water - YouTube. Spelling counts A B Calculate the electronegativity difference of the Si-S bond.
The structure on the right is the Lewis electron structure or Lewis structure for H 2 O. In panel a the skeletal structure for water is shown with the atoms represented by their Lewis symbols. With two bonding pairs and two lone pairs the oxygen atom has now completed its octet.
1 Get Other questions on the subject. Therefore the other 4 electrons must go on the oxygen. What is the molecular geometry for this molecule.
A Consider the Lewis-dot structure for SiSHI. We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure Sulfur trioxideFor the SO3 structure use the periodic table to find the total number.
Lewis dot diagram for water. The structure on the right is the Lewis electron structure or Lewis structure for H2O. A step-by-step explanation of how to draw the BF3 Lewis Dot Structure Boron TrifluorideFor the BF3 Lewis structure calculate the total number of valence.
Unpaired electrons on the central atom will bend the atom. SO2 Lewis structure sulfur dioxide electron dot structure is that type of diagram where we show the total 18 valence electrons of SO2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. Moreover by sharing a bonding pair with oxygen each hydrogen atom now has a full valence shell of two electrons.
This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons and each H atom having two valence electrons.

Lewis Dot Structure Easy Hard Science

Lewis Structure Practice Worksheet 4 Stepsa Ch4 Lewis Structure In 2020 Practices Worksheets Graphing Linear Equations Chemistry Worksheets

Lewis Dot Structure Easy Hard Science

Lewis Electron Dot Structures Detailed Explanation With Examples Videos

Lewis Dot Structure Easy Hard Science

Mgcl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

How To Determine The Lewis Dot Structure For Methane Quora

Co2 Lewis Structure Carbon Dioxide In 2021 Carbon Dioxide Lewis Molecules
Does It Matter Where You Put The Dots On A Lewis Structure Quora

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Does It Matter Where You Put The Dots On A Lewis Structure Quora

Lewis Dot Structure Easy Hard Science

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Lewis Dot Structure Easy Hard Science
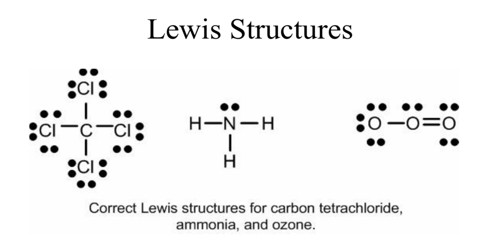
Limitations With Lewis Structures Qs Study

H2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
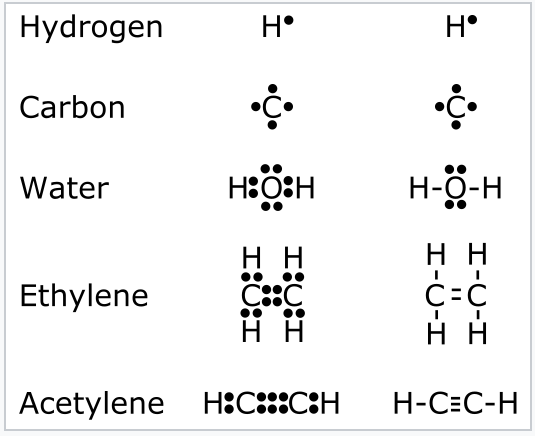
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts

Lewis Dot Structure Easy Hard Science

How To Draw A Lewis Structure Lewis Chemistry Draw