How Many Valence Electrons Does C2h4 Have
Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Valence electrons are the electrons in the outermost shell or energy level of an atom.

Ethylene American Chemical Society Chemical Chemistry Society
It is a simple alpha particle called helium particle.
How many valence electrons does c2h4 have. The symbol for the helium element is He. After the electron configuration the last shell of the boron atom has three electrons. The atomic number of carbon is 6.
For example oxygen has six valence electrons two in the 2s subshell and four in the 2p subshell. Helium atoms do not usually do this. For the SO3 2 compound we have 26 total valence electrons and that includes these two electrons up herethere are two extra valence electronsSo we have 26.
The Group 3 atoms have 3 valence electrons. In this regard can nitrogen have 8 valence electrons. The two sp2 hybrid orbitals get overlapped by two hydrogen atoms containing unpaired electrons.
There are 2 types of elements in the periodic table. How many valence electrons does magnesium have. The atomic number is the number of protons.
Similar to other elements too the users can find out the valence electrons. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. How many valence electrons does nitrogen have.
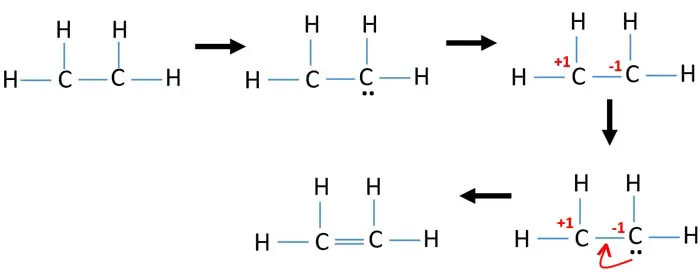
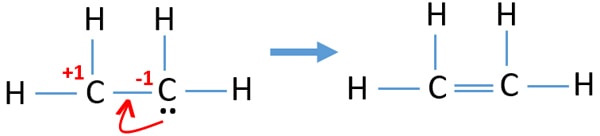
Electrons equal to protons are located in a circular shell. Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond. How Many Valence Electrons Does Lead have.
During radioactivity some of the particles emitted consist of 2 neutrons 2 protons and 2 electrons. Protons and neutrons are located in the nucleus. The elements that have 1 2 or three electrons in the last shell donate the electrons in the last shell during bond formation.
Subsequently question is does oxygen have 5 valence. The total number of valence electrons is 5611. The Group 4 atoms have 4 valence electrons.
Thank you for the question. A pi bond is formed by the unhybridized 2pz orbitals of each carbon atom. Ions are charged when they lose NS electrons.
Magnesium has just two valence electrons and 12 electrons. 455 1145 Views. For C2H4 you have a total of 12 total valence electrons.
Click to see full answer. This tendency is called the octet rule because each bonded atom has 8 valence electrons including shared electrons. Helium does not participate in.
As a general rule a main group element except hydrogen or helium tends to react to form a s2p6 electron configuration. Oxygen needs 2 more electrons to fill its outermost shell. That is the number of protons in the carbon is 6.
Compounds do not posses valence electrons. We know the details about this. How many valence electrons are present in the electron configuration.
Now if we talk about the element lead or Pb then there are four 4 electrons on the outer shell or the 6 th shell therefore the number of valence electrons that lead has is 4. C2H4 is an organic compound named Ethene. Given this how many electrons are in each element.
The carbon atom consists of 6 electrons and hydrogen has 1electron. Helium atoms do not form ions. When we say or talk about the valence shell electrons it could be found out as the total number of electrons that are there in the outer shell of any molecule or an atom.
Sodium will loose 1 electron to form ion. When we look at the molecules of C2H4 it has 2 CH molecules and 4 H molecules. 14 Votes In the Lewis structure of C2O42- there are a total of 34 valence electrons.
See full answer. The valence electrons of helium are two and the total number of electrons is two. The nucleus is located in the center of the atom.
Therefore no matter how electrons are shared between the nitrogen and oxygen atoms there is no. As you can see oxygen has 2 of its 8 electrons in the shell closest to the nucleus and the remaining 6 electrons - which are called valence electrons - in its second shell - this is oxygens outermost shell. In this case both the valence and valence electrons of boron are 3.
Nitrogen has a total of 5 valence electrons so doubling that we would have a total of 10 valence electrons with two nitrogen atoms. Magnesium is classified as a metal. Metals are the elements which have a tendency to loose electrons and thus they form cations.
How many valence electrons of boron ion have. How many valence electrons does californium have. 15 How many pi bonds does c2h4 have.
How Many Valence Electrons Does Helium Have How many valence electrons are there in helium ion. How many electrons and protons does a carbon atom have. By signing up youll get thousands of step-by-step solutions to your homework.
Beryllium has two valence electronsWhat makes a particular element very reactive and another element non-reactive. Correspondingly how many valence electrons does so32 have. Lets put the Sulfur at the center and the Oxygens around the outside.
The elements that form bonds by. The Group 2 atoms have 2 valence electrons. The Group 1 atoms have 1 valence electron.

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry Shape And Bond Angles Youtube

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

Welcome To Learnapchemistry Com Bond Length Molecular Geometry Covalent Bonding
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
Lewis Structure Of C2h4 Biochemhelp