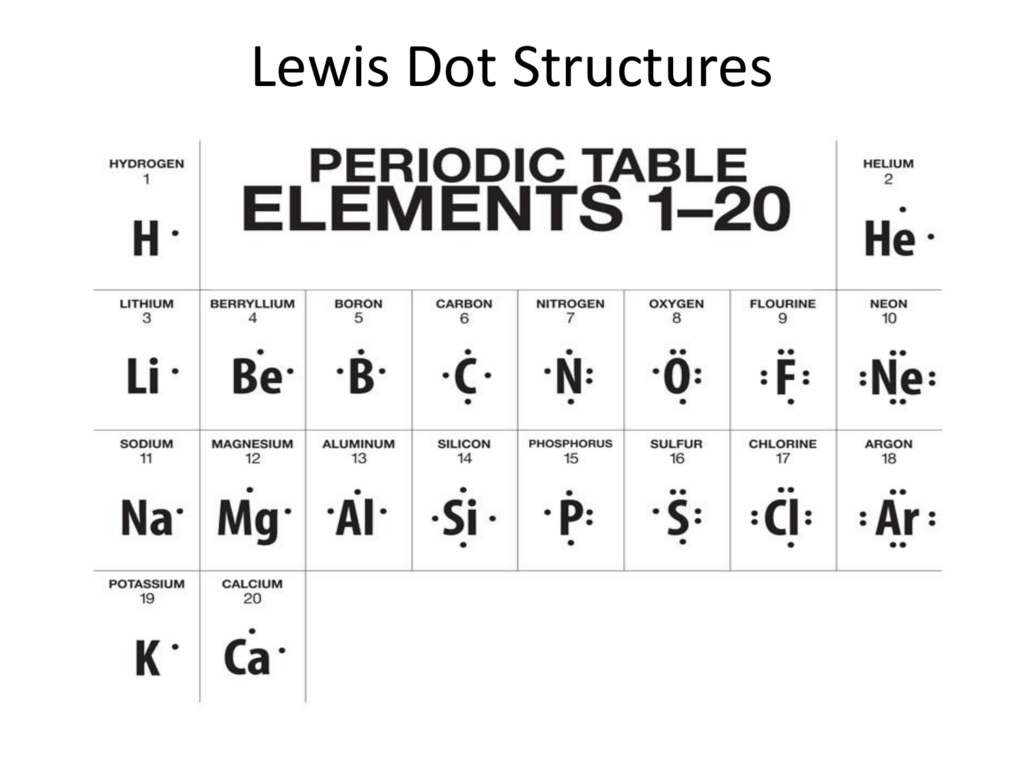
What Is The Lewis Dot Structure Of H2o
Lewis sturucture is the one in which an atom or molecule is represented in the form of dots. In the lewis structure of H 2 O there are two single bonds around oxygen atom.
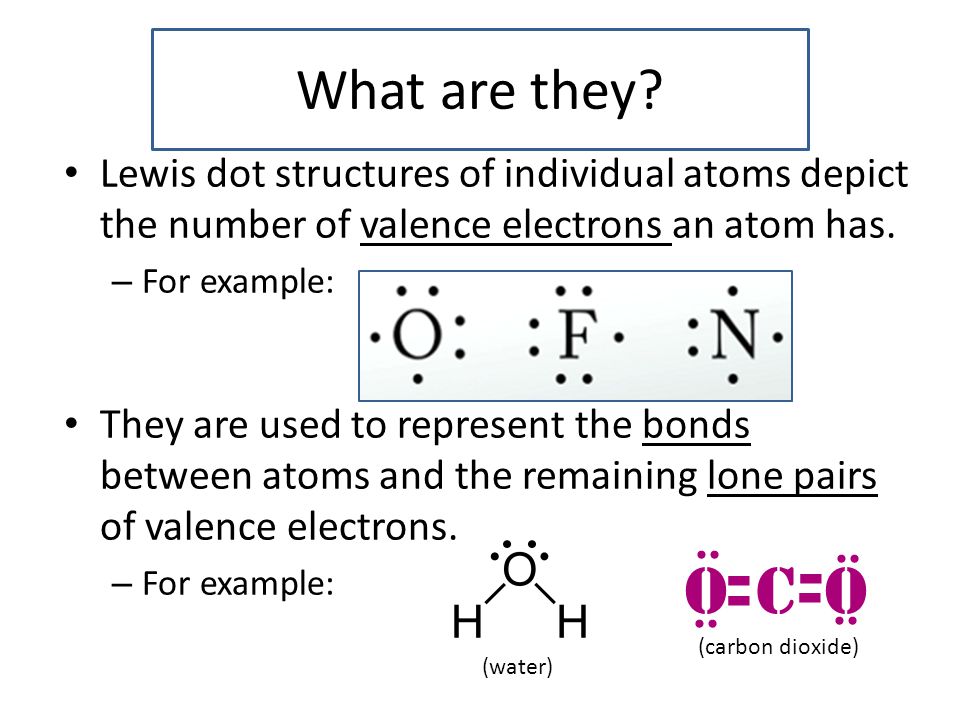
Lewis Dot Structures Ppt Video Online Download
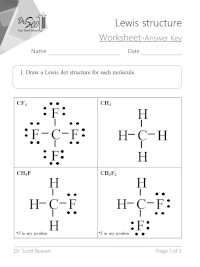
A step-by-step explanation of how to draw the H2O Lewis Dot Structure WaterFor the H2O structure use the periodic table to find the total number of valenc.
What is the lewis dot structure of h2o. You can find a procedure for drawing Lewis structures at this location. Both Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure. For H₂O O must be the central atom The skeleton structure is H-O-H.
The Lewis electron dot structures of a few molecules are illustrated in this subsection. H2O Lewis structure water electron dot structure is that type of diagram where we show the total eight valence electrons of H2O as dots or dots and dashes -In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots. CO2 lewis dot structure contains two oxygen atoms and one carbon atom connected with the double bond whereas carbon is the central atom and no lone pair is present on it.
The central atom of this molecule is carbon. On the right and left sides are a singly bonded H atom. O has 6 valence electrons and each H has one.
Water is in fact a chemical. As a result there are two lone pairs in this molecule and two bonding pairs of electrons. Since it is bonded to only one carbon atom it must form a double bond.
Lewis structure of water molecule contains two single bonds around oxygen atom. Water or H2O H 2 O has the electron dot structure shown below. The Lewis dot structure of water begins with a single O atom in the center.
Lewis Structure of H2O. Lewis Structure of CO2. Secondly what is the formula of h2o.
Hydrogen atoms are joint to oxygen atom through. A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure Hydrogen peroxide. The two dots ie the electrons betw.
For example the lewis structure of NaCl will be represented as given below. H2Os Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. Electrons of the covalent bond by two dots.
But each oxygen in the CO2 lewis dot structure has two lone pairs. Its chemical formula is H2O or less commonlyHOH when properly written the 2 after the H is written. Look for the total valence electrons.
The other four valence electrons in oxygen are in pairs at the bottom. Each step of drawing lewis structure of H 2 O are explained in this tutorial. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2s linear structure.
In order to eject an electron from a metal a photon of a certain minimum energy must strike the sur. Each dot represent an electron in an atom. Lewis Structure Examples.
This bent molecular structure gives it many unique properties such as being polar. H 2 O lewis structure. Oxygen contains 6 valence electrons which form 2 lone pairs.
This is the Lewis structure of the H 2 O molecule that has two single bonds between Oxygen and Hydrogen. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond.
For showing the sharing of electrons show a single bond on both sides. The structure must have a total of 8 valence electrons because there are 2. One of the most fascinating phenomena is the idea of hydrogen.
The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Similarly one may ask what is the Lewis dot structure of water.
Here we need to understand how the Lewis structure is drawn for the H2O molecule. It is eight to form a single H2O molecule. I quickly take you through how to draw the Lewis Structure of water H2O.
Note that the H2O2 Lewis structure is frequently used on tests a. 12 Electron Dot Structure Of H2O. A step-by-step explanation of how to draw the H2O Lewis Dot Structure WaterFor the H2O structure use the periodic table to find the total number of valenc.
There are two hydrogens that bind with 1 electron from those 6 so there are 2 electrons that are binding with hydrogen. I also go over hybridization shape and bond angle.
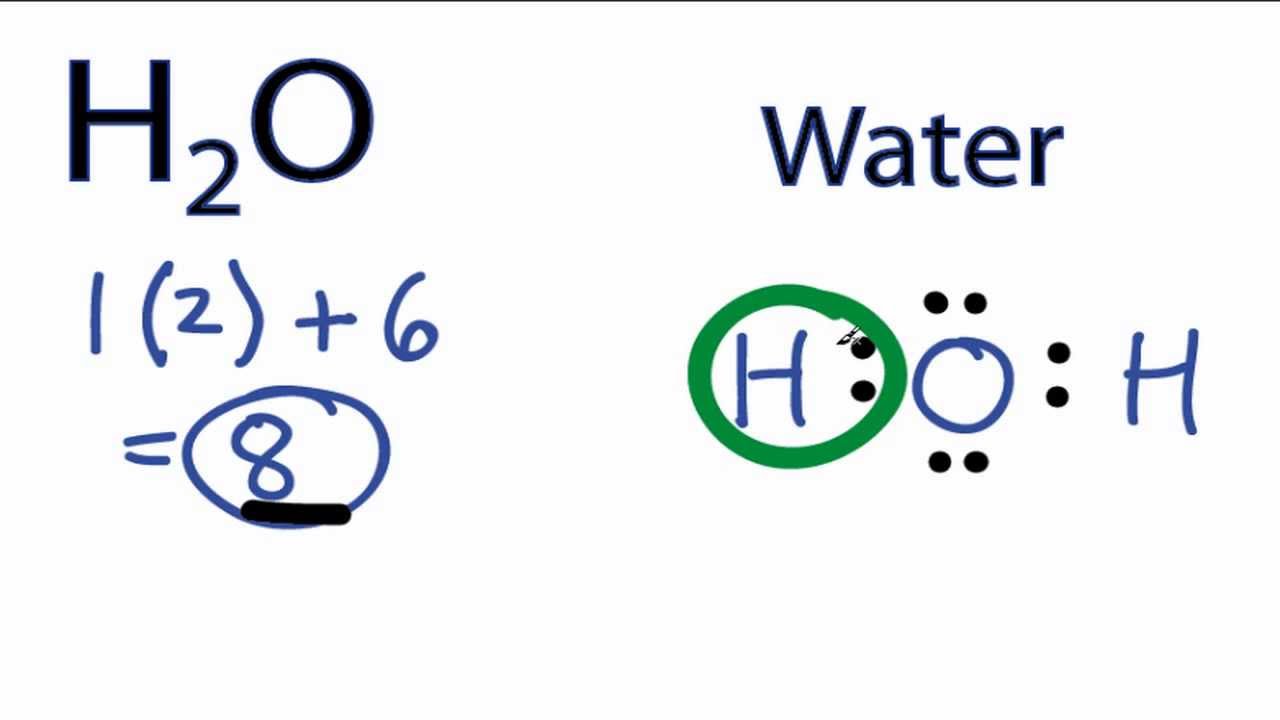
Water Lewis Structure How To Draw The Lewis Structure For Water Youtube
How To Draw The Lewis Structure Of 2 H2o On Paper I Need It For The Exam Quora

Lewis Dot Structure Easy Hard Science
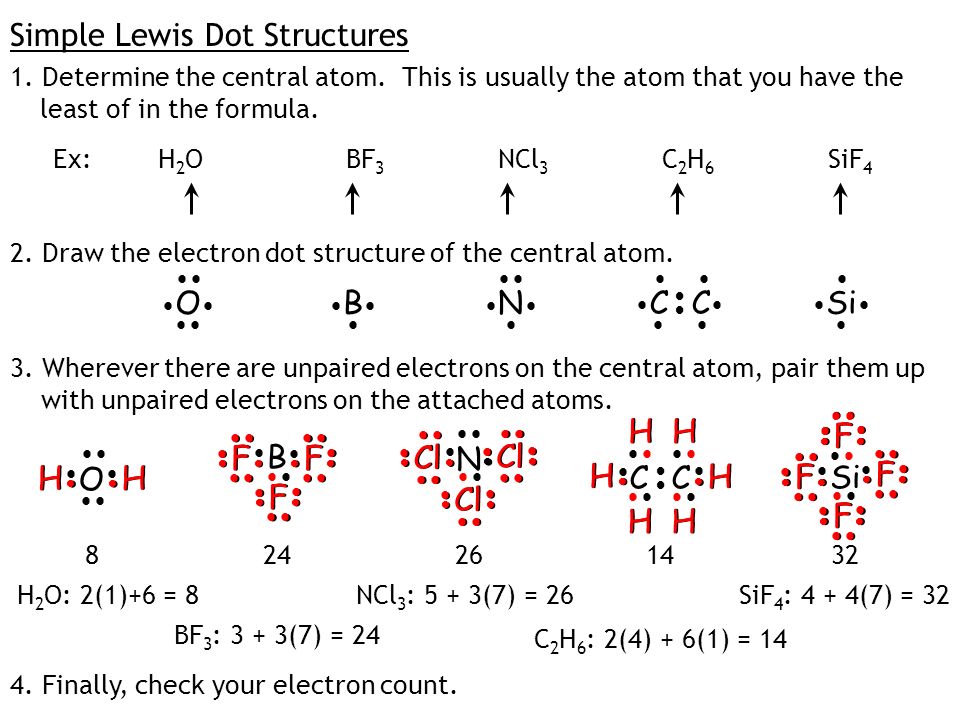
Lewis Dot Structures Electron Dot Structures Of Compounds Ppt Download

Makethebrainhappy The Lewis Dot Structure For H2o
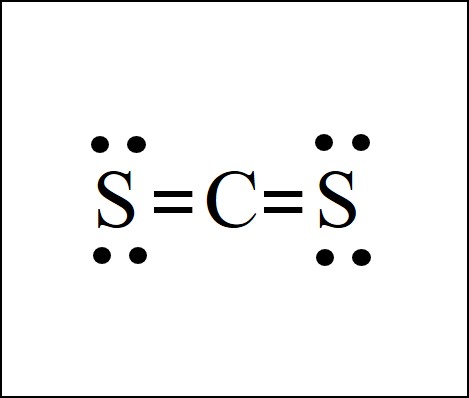
Lewis Dot Structure Easy Hard Science

H2o Lewis Structure Water Youtube
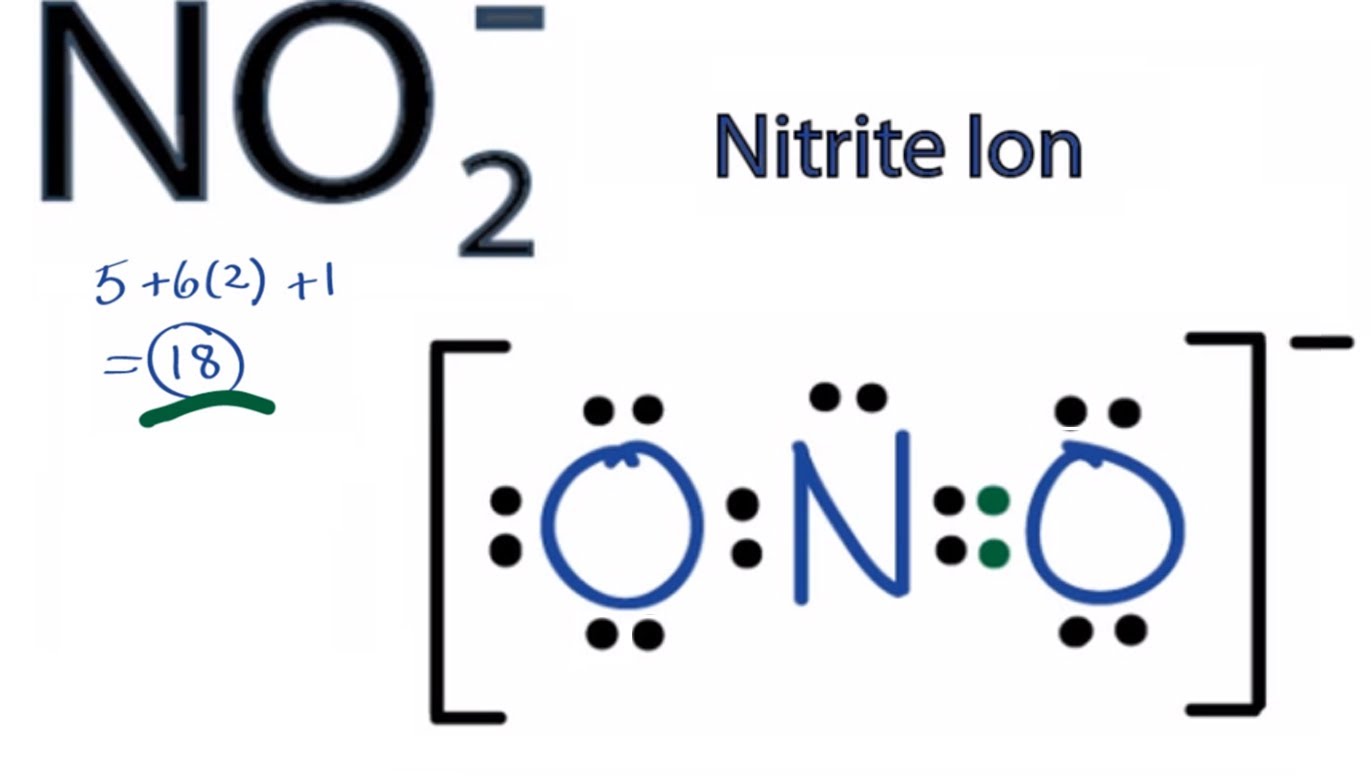
Drawing Lewis Structures Chemistry Socratic

Makethebrainhappy The Lewis Dot Structure For H2o
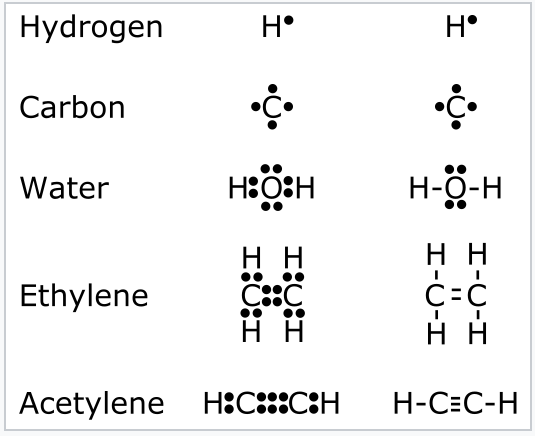
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts

H2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
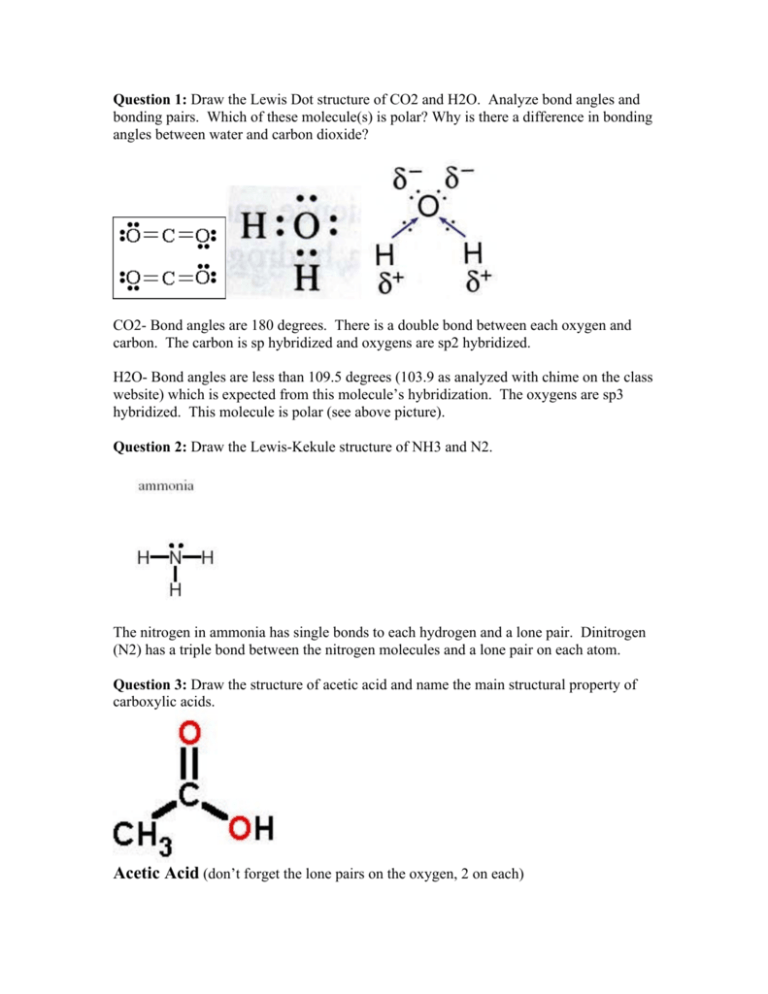
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube

How To Determine The Lewis Structure For H2c2o4 Quora

How To Draw A Lewis Structure Lewis Chemistry Draw

Makethebrainhappy The Lewis Dot Structure For Ch4

Lewis Structure Simple English Wikipedia The Free Encyclopedia
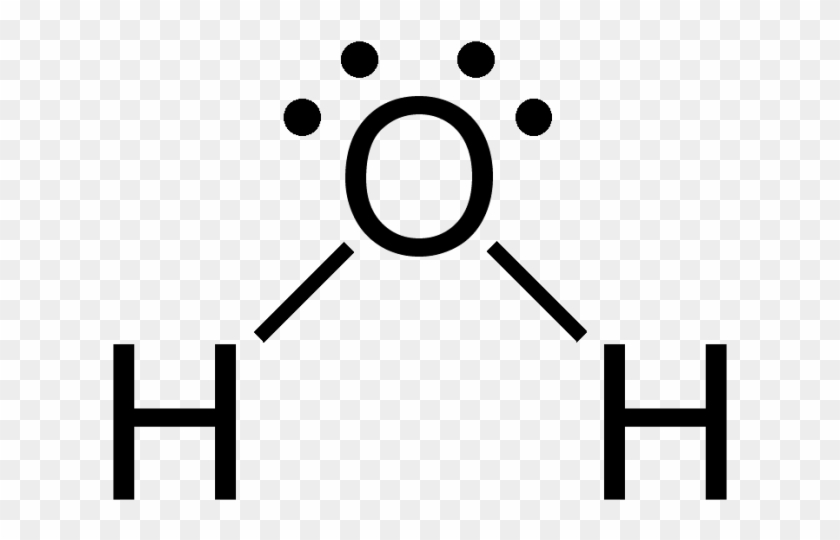
A Lewis Dot Structure Showing The Electron Configuration Water Lewis Structure Free Transparent Png Clipart Images Download