Xef4 Lewis Structure Dipole Moment
The Lewis structure for XeF4 has a total of 36 valence electrons. BCl3 is a trigonal planar which the forces would be balanced.

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules
What type of bond is XeF4.

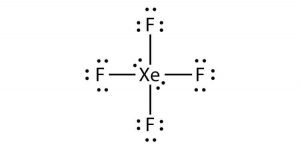
Xef4 lewis structure dipole moment. Net dipole moment. Polarities of Xe-F bonds is zero as they cancel out each other. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table.
As there are four electrons on the Xenon atom which are localized as nonbonding pairs of electrons. General Chemistry -- Dipole MomentView the complete course. The individual Xe-F bonds are polar due to unequal electronegativity of Xe and F atoms but the net vector sum of the polarities of Xe-F bonds is zero as they cancel out each other.
So the net dipole moment of XeF4 is 0 Debye. Xef4 Dipole Moment Which element between If5 Xef4 Sf6 and CH4 dipole moment. Measurements of Radziemski et al.
Although the bonds between Xenon and Fluorine atoms are polar XeF4 is a nonpolar molecule. All the polar molecules have a net dipole moment and all nonpolar have zero dipole moment. Although the bonds between Xenon and Fluorine atoms are polar XeF4 is a nonpolar molecule.
Here in this post we described step. Electronegativity difference 398 260 138 The difference is pretty significant. A three-step approach for drawing the SCl4 Lewis structure can be used.
The second step is to valence electron to the four chlorine atoms and the final step is to combine the step1 and step2 to get the SCl4 Lewis Structure. However I dont know the other two - AsH3 and SCl3 which one is with dipole dipole attraction between molecules. Dipole moment Charge Q distance of separation d Its unit is Debye and denoted by D.
As there are four electrons on the Xenon atom which are localized as nonbonding pairs of electrons. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. The Lewis structure for XeF4 has a total of 36 valence electrons.
When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. In each case the individual bond dipoles cancel out leaving the molecules nonpolar. Learn to determine if CF4 is polar or nonpolar based on the Lewis Structure and the molecular geometry shape.
This is an accurate way to determine whether ClF3 is polar or non-polar. When we are having equal sharing electrons in molecules then there is no difference in electronegativity due to equal sharing. According to structure the center atom is carbon and four hydrogen is bound to it.
A covalent molecule can be polar or non-polar depending on the types of bonding. In this case dipole moment. Although the Xe-F bonds themselves are polar covalent the symmetrical arrangement of these bonds results no net dipole moment cancellation of the polar vectors.
The 4 F atoms are arranged around the Xe atom with bond angles of 90 degree. XeF4 is with tetrahedral shape where one side is a lone pair which the charge is imbalanced and it has dipole dipole attraction between molecules. Xenon tetrafluoride XeF4 is a non-polar chemical compound owing to its symmetrical square planar structure.
UCI Chem 1A General Chemistry Winter 2013Lec 11. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Since it is asymmetrical the molecule is polar and has a net dipole moment.
So the net dipole moment of XeF4 is 0 Debye. Drawing CH3Br Lewis Structure is very easy to by using the following method. 1D 33356410-30 Cm where C is Coulomb and m is meter.
Polarity of XeF4 Bond Polarity of Xe-F The electronegativity of Xenon is 26 and that of fluorine is 398. This means that the individual Xenon-Fluorine Xe-F bonds are polar in nature. When there is no difference of electrons there was no polarity.
The Attempt at a Solution. The bromomethane chemical formula is CH3Br. The higher the dipole moment of the molecule greater is the polarity strength of that molecule.
All the Xe-F bonds are in opposition with each other mutually making the sum of dipole moment zero. Carbon bonds are formed with four hydrogen and all hydrogen have equal sharing electron with carbon. XeF4 has a symmetrical square planar shape.
The molecule is therefore non-polar. If the molecule has some net dipole moment then that molecule is polar in nature. Key Points To Consider When Drawing The SCl4 Structure.
Thus it is a neutral substance. XeO4 is tetrahedral and XeF4 is square planar and both are symmetrical. The dipole moment is the major aspect for any compound to be polar or nonpolar.
The first step is to sketch the Lewis structure of the SCl4 molecule to add valence electron around the sulfur atom. All the Xe-F bonds are in opposition with each other mutually making the sum of dipole moment zero. Therefore there is no net dipole moment.

How To Draw Xef4 Lewis Structure Science Education And Tutorials

Is Xef4 Polar Or Nonpolar Techiescientist

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Is Xef4 Polar Or Nonpolar Techiescientist
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Is Nh3 Polar Or Nonpolar Vsepr Theory Molecules Polar

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Is Xef4 Polar Or Non Polar Xenon Tetrafluoride Youtube

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Is Xef4 Polar Or Non Polar Xenon Tetrafluoride Youtube

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Is Xef4 Polar Or Non Polar Xenon Tetrafluoride Youtube