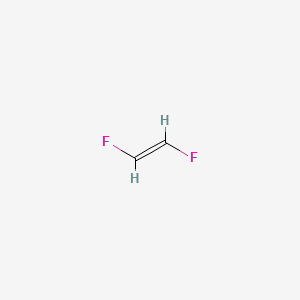
Lewis Structure For C2h2f2
Calculate the total valence electrons in the molecule. The Answer is linear.
1 2 Difluoroethylene C2h2f2 Pubchem
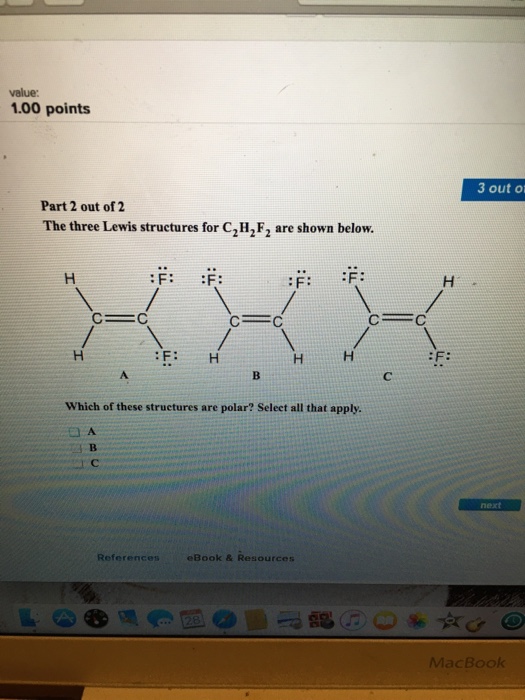
Draw three Lewis structures for compounds with the formula C 2 H 2 F 2.
Lewis structure for c2h2f2. PO 4 3 b. Global production in 1999 was approximately 33000 metric tons. It is a flammable gas.
Give the hybridization of one of the carbon atoms. 6 rows 12-Difluoroethylene C2H2F2 CID 5365501 - structure chemical names physical and chemical. To do so we first need to do the following steps.
A single bond in a Lewis structure represents 2 electrons. It looks like a lewis dot structure for C2H2F2. What is the molecular geometry of trans-difluroethylene.
CO 3 2 4. Put two canbon atoms in the center side by sidePut one fluorine on each carbon atom. Give the hybridization of one of the carbon atoms.
Alternatively a dot method can be used to draw the lewis structure of C 2 F 2. Draw Lewis Structure for 1st isomer. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Br 2 Lewis structure.
A bonding orbital for C1-C2 with 19967 electrons __has 5000 C 1 character in a sp138 hybrid. For the molecule we expect the carbons to be the central atoms in this molecule since carbon tends to be the central atom in its compounds. Select all the correct answers.
The hybridization of the atoms in this idealized Lewis structure is given in the table below. Indicate which of the compound s are polar. Draw the Lewis structure for the molecule.
Sanchez LG Polarity of the Gas Chromatographic Stationary Phases and Retention Indices of. Draw the Lewis dot structure for each of the following polyatomic ions. A Draw all possible resonance Lewis structures.
Calculate the total number of valence electrons present. How do you figure out the answer is linear. Give the molecular geometry corresponding to one of the carbon atoms.
In the Lewis structure for C 2 H 2 Br 2 there are a total of 20 valence electrons. Give the molecular geometry corresponding to one of the carbon atoms. I think its a tetrahedral.
CH 2 Br 2 d. Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar. It is primarily used in the production of fluoropolymer s such as polyvinylidene fluoride.
Determine the central atom in this molecule. Is the molecule polar Yes or No. If all forms are equivalent circle none.
NH 4 c. H 2 S c. 13-Difluoropropadiene C3H2F2 CID 12590904 - structure chemical names physical and chemical properties classification patents literature biological.
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. Best Lewis Structure The Lewis structure that is closest to your structure is determined. Hybridization in the Best Lewis Structure.
A SO 2 b CH 3CONH 2 connectivity as shown below c NCO C is central 3. What is THE LEWIS DOT STRUCTURE FOR C2H2F2. On a clean sheet of paper draw the Lewis electron structure for C2H2F2 12 trans a.
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. Tell me about the best Lewis structure. A single bond in a Lewis structure represents 2 electrons.
I know you draw the lewis structure but when I drew it I thought. I know how to draw the lewis structure but Im not sure how to find the molecular geometry. NO 3 d.
Hybridization in the Best Lewis Structure. On a clean sheet of paper draw the Lewis electron structure for C2H2F2 12 trans a. Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene C2H2.
A bonding orbital for C1-C2 with 19965 electrons __has 5000 C 1 character in a sp139 hybrid. Draw the Lewis dot structures for each of the following molecules. Remember that Hydrogen H atoms always go on the outside of a Lewis Structure.
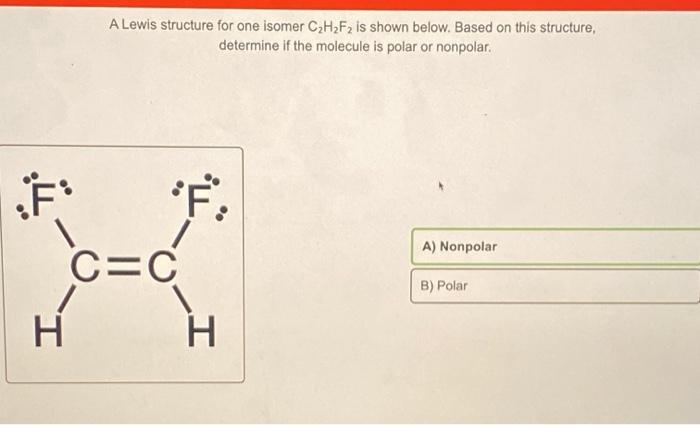
11-Difluoroethylene also known as vinylidene fluoride is a hydrofluoroolefin. Draw the Electron dot structure. If the molecule is polar on the Lewis structure above clearly.
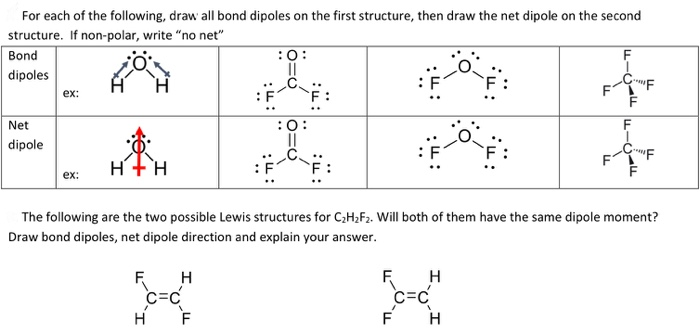
The valency of carbon is four so it likes to have exactly four bonds so if the each carbon atom is bonded to two non - carbon atoms the. The following are the two possible Lewis structures for C 2H 2F 2. Tioderbo c2h2f2 lewis dot structure c2h2f2 lewis dot structure - Hcg failure Tell me about the atomic charges dipole moment bond lengths angles bond orders molecular orbital energies or total energy.
For the following molecules or ions where the central atom is underlined. B Assign formal charges for all atoms in each resonance structure. C Circle the favored resonance form.
The hybridization of the atoms in this idealized Lewis structure is given in the table below. Note that Hydrogen only needs two valence electrons to have a full outer shell. Estimate the H-C-F bond angle d.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. Estimate the H-C-F bond angle d. Will both of them have the same.
The Three Lewis Structures For C 2h 2f 2 Are Shown Chegg Com
Https Blogs Nvcc Edu Alchm Files 2019 05 111 11lewisstructure2summer2019 Pdf

For Each Of The Following Draw All Bond Dipoles On Chegg Com
Https Blogs Nvcc Edu Alchm Files 2020 04 111 11lewisstructuresspring2020 Pdf

Ch3conh2 Lewis Structure Ch3conh2 Polar Or Nonpolar

C2h2br2 Lewis Structure How To Draw The Lewis Structure For C2h2br2 Youtube

Covalent Problem C2h2f2 Youtube
Https Blogs Nvcc Edu Alchm Files 2019 05 111 11lewisstructure2summer2019 Pdf
Write The Formula For Nitrous Acid 3 N 02 03 1 1 2 3 Chegg Com
Z 1 2 Difluoroethylene C2h2f2 Pubchem
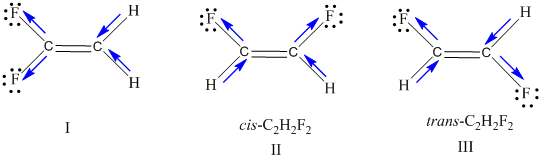
Solved Draw Three Lewis Structures For Compounds With The Formula Chegg Com
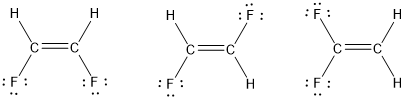
Solved Draw Three Lewis Structures For Compounds With The Formula Chegg Com

Xef2 Lewis Structure Xef2 Xenon Difluoride
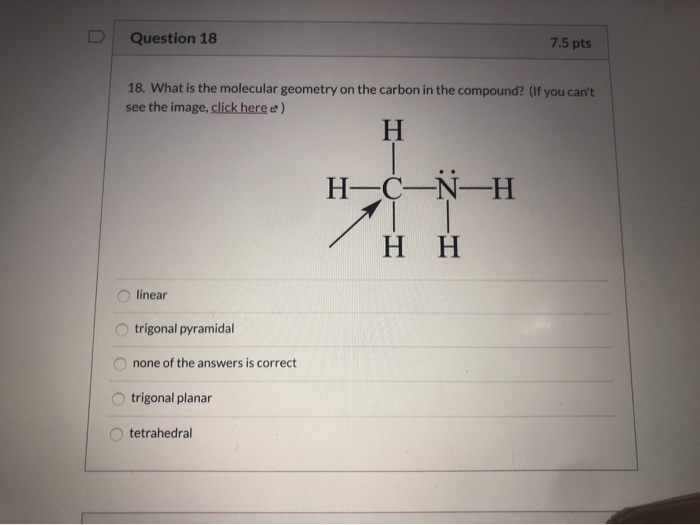
Question 16 7 5 Pts 16 What Is The Total Number Of Chegg Com
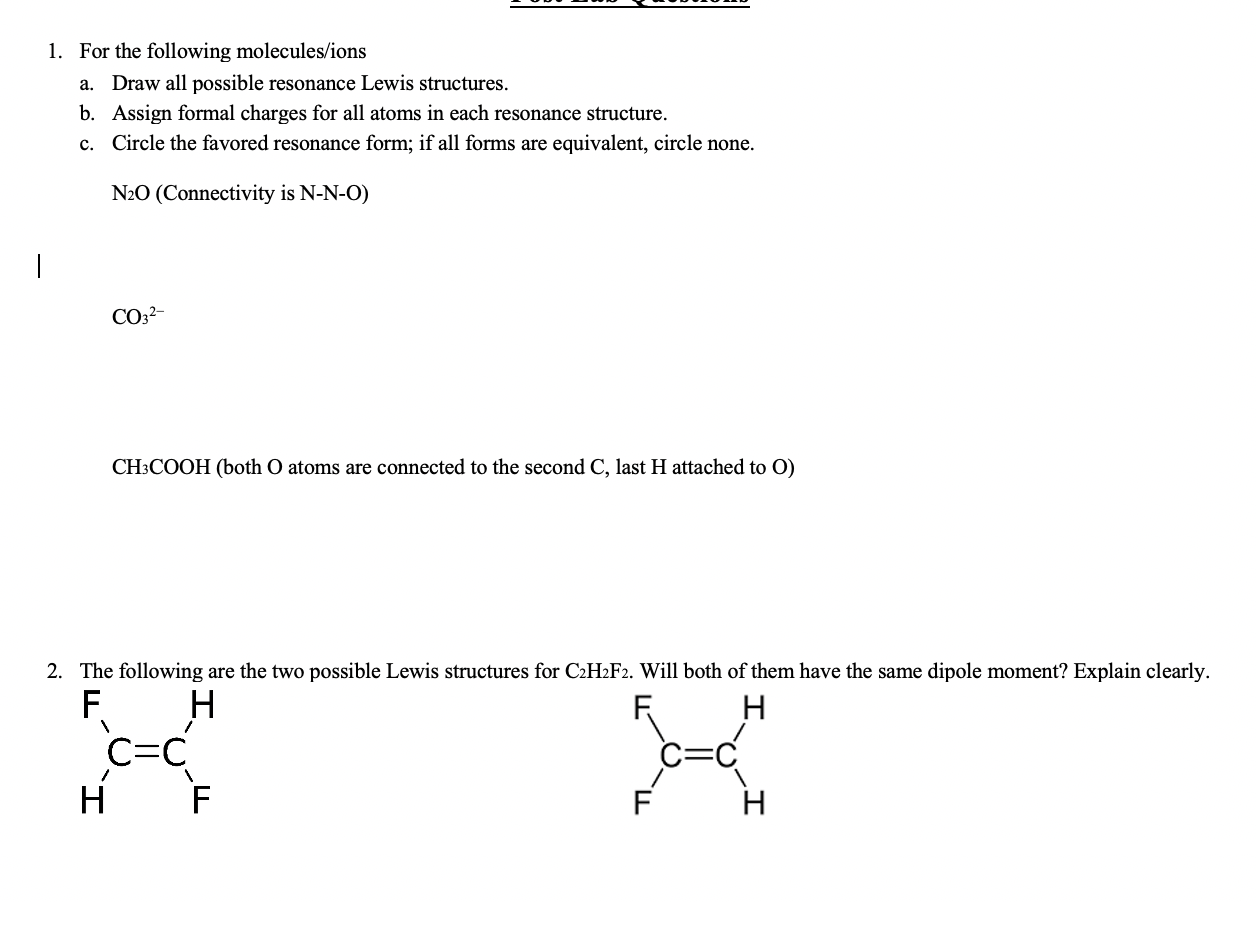
1 For The Following Molecules Ions A Draw All Chegg Com
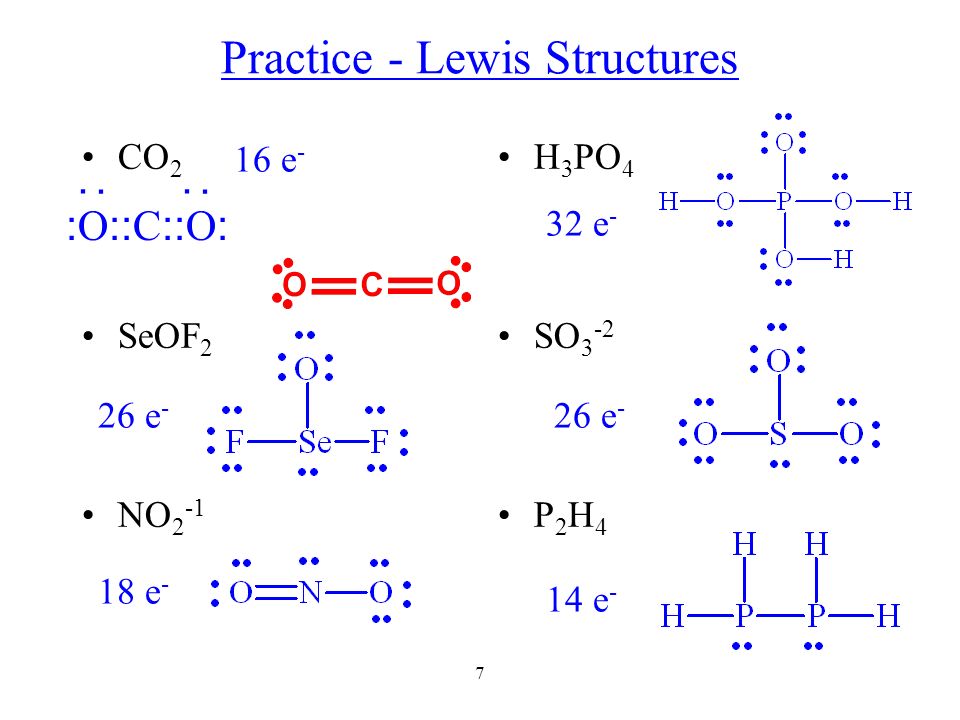
1 Chemical Bond Ionic Bond 4 Types Of Bonds Covalent Bond Ppt Video Online Download
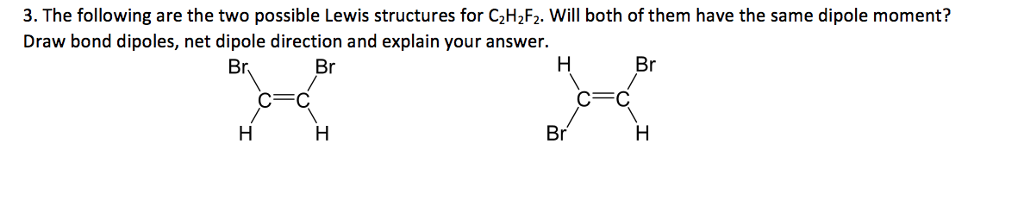
3 The Following Are The Two Possible Lewis Chegg Com

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

Covalent Problem C2h2f2 Youtube