Lewis Structure Of Pcl4+
This is an AX₄ ion. Tetrachloroboranuide BCl4- CID 5326109 - structure chemical names physical and chemical properties classification patents literature biological activities.

How To Draw The Lewis Structure Of No2 Nitronium Ion Lewis Chemistry Help Study Tips
In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond.

Lewis structure of pcl4+. Show the formal charges of all atoms in the correct structure. The lewis dot structure for ccl4 starts with a c in the middle. Drawing the Lewis Structure for PCl 4-Viewing Notes.
17 has 17 protons and 17 electrons so overall no change. The phosphorus has an electronegativity value of 219 and chlorine comes with 316. In the Lewis structure for PCl 4-there are a total of 34 valence electrons.
Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line marked at the end of which is a set of electrons. Four pairs will be used in the chemical bonds between the P and F. These structures are called resonance structures.
Draw the Lewis structures for the reactants and products of PCl4 with Cl-. If you add up all of the electrons in hypothetical PCl 4 15 17 17 17 17 83-. Identify the Lewis acid and the Lewis base in the reaction.
And CS2 Draw the Lewis structure. Include all lone pairs of electrons. Predict the electron pair geometry and the molecular structure of eqPCl_4- eq.
Draw the Lewis Structure for PCl4 and indicatethe number of electron domains around the central atom. Phosphorus uses sp³ orbitals in PCl₄. Drawing the Lewis Structure for PCl 5.
It has 4 bonding pairs and no lone pairs. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. The bonds should point toward the corners of a regular tetrahedron.
So hypothetical PCl 4 has a charge 83 - 83 0. A combination of all these resonance structures represents the real or observed structure. For such a molecule several dot structures may be drawn.
Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. In this tutorial we will learn how to draw the lewis structure of PCl 3 with all theories. Tetrachlorophosphanium Cl4P CID 643599 - structure chemical names physical and chemical properties classification patents literature biological activities.
A Cl- is lewis acid PCl4 is the base. PCl4 NO3- O3 trans - CCl2F2 are non polar. Use VSEPR Theory to predict the orbital geometry.
Each chlorine at no. PCl4 has 32 valence electrons P is the central atom The question is. 10 Pcl4 Lewis Structure.
Structures contribute to the electronic structure of the molecule. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. You can check by drawing out the lewis structure for each.
Learn to determine if CCl4 is polar or nonpolar based on the Lewis Structure and the molecular geometry shape. In the PCl 4-Lewis structure Phosphorus P is the least electronegative so it goes in the center. The shapes of P C l4P C l4 and AsC l5 are tetrahedral see-saw and trigonal bipyramidal respectively.
Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms. Once we know how many valence electrons there are in PCl5 we can distribute them around the central atom and attempt to. If you add up all of the protons in hypothetical PCl 4 15 17 17 17 17 83.
Lewis structure of a compound is the arrangement of its underlying atoms valence shell electrons. Draw the Lewis structure. Predict the electron pair geometry and the molecular structure of PCl4-.
Since Iodine I is below Period 3 on the periodic table it can hold more than 8 electrons. Draw the molecules connecting them with bonds. Lewis Structure of PCl5.
PCl4 Cl- PCl5. Also there is a lone pair on phosphorus atom. In the Lewis structure of ICl4- there are total of 36 valence electrons.
Thus the trial structure is the correct lewis structure. PCl 4-is a negative ion an anion so you have to add an extra valence electron. PCl 3 Phosphorus Trichloride Lewis Structure.
The Lewis structures of some molecules do not agree with the observed structures. Answer Pcl4 is Nonpolar What is polar and non-polar.

Drawing The Lewis Structure For Pcl4 Youtube
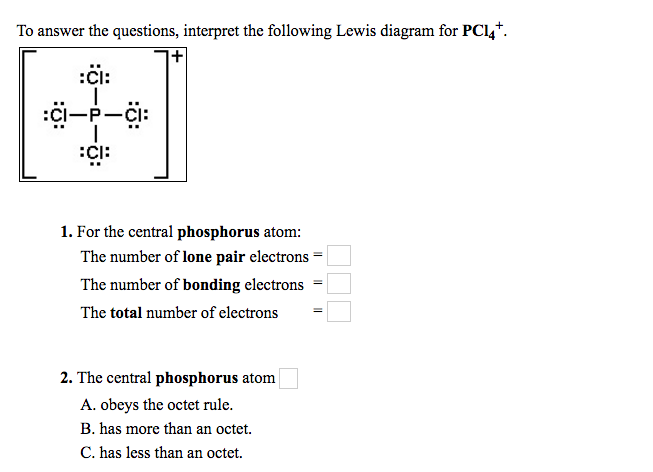
To Answer The Questions Interpret The Following Chegg Com

Nocl Lewis Structure Nitrosyl Chloride Youtube

How To Draw The Lewis Dot Structure For Pbr4 Youtube

126 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube Chemistry Classroom Science Chemistry Chemistry

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry

How To Draw The Lewis Structure For Xef3 Youtube

Pcl4 Lewis Structure How To Draw The Dot Structure For Pcl4

How To Draw The Lewis Dot Structure For Hpo4 2 Hydrogen Phosphate Ion Youtube

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Pcl6 Lewis Structure How To Draw The Lewis Structure For Pcl6 Youtube

Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles Molecular Geometry Molecular Bond

How To Draw The Lewis Structure For Pcl4 Youtube
Day02 3 Lewis Structure H2co3 Hco3 Pcl4 Youtube

How To Draw The Lewis Dot Structure For Pbr4 Youtube

126 Clo4 Lewis Structure How To Draw The Lewis Structure For Clo4 Perchlorate Ion Youtube Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Lewis Representation Of Simple Molecules Lewis Structure Chemistry For Class 11 In Hindi Youtube

