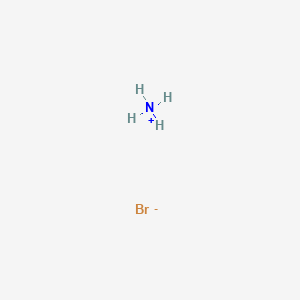
Nh4+ Lewis Structure Hybridization
There have four electron containing orbital in outer most shell of N atom. MgF2 Lewis Structure Geometry Hybridization and Polarity Magnesium fluoride or MgF2 is a stable non-hygroscopic compound that is merely soluble in dilute acids and insoluble in alcohol.
Nh4 Ammonium Ion Lewis Structure
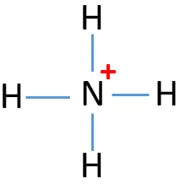
A positively charged polyatomic ion of Ammonium or NH4 comes into existence when an Ammonia atom goes through the process of protonation that is it loses one of its electrons and becomes positively charged.
Nh4+ lewis structure hybridization. In NH4 ion there is no pi bond. How many valence electrons are in NH4. It is a stable hydride formed of one nitrogen and three hydrogen atoms.
According to VSEPR theory the shape on an ammonium ion NH4 is most similar to If you were to construct the molecule H2O using a molecular kit. Ammonia is a colorless compound used in making fertilizers. Nonpolar molecule b Lewis.
WHAT IS THE HYBRIDIZATION OF CARBON IN METHANE CH 4. What is the hybridization on the central carbon atom in CCl2F2. To find the hybridization for NH4 well first determine the steric number.
Nh4 bond angle. If you are confused about how the number of orbitals becomes four then its simple that ammonia molecule has three sigma bonds and one lone pair of. Also remember that the valency of hydrogen is one.
Since NH4 is a cation the bond angle between 2 respective hydrogen atoms is 1095 degrees instead of 90 degrees which is as far away from one another as possible. AB 3 trigonal planar 3-D sketch. Draw the Lewis structure for NH3.
MOLECULE NAME LEWIS STRUCTURE SHAPE HYBRIDIZATION BOND POLARNON SIGMA NAME ANGLE POLAR PI BONDS H2O CH4 NH4 SO3. All electron containing atomic orbital of outer most shell of N atom are participate in hybridization. A quick explanation of the molecular geometry of NH4 including a description of the NH4 bond anglesLooking at the NH4 Lewis structure we can see that the.
As a result hybridization of central N atom is sp3. This VSEPR package use the Jmol molecule viewer. Write Lewis structures for the following.
Write Lewis structures that obey the octet rule for each of the following. What is the shape of NH4. Write Lewis structures for the following molecules or ions which have central atoms that do not obey the octet rule.
It can form an NH4. There is a best shortcut method to find hybridisation first count sorounding atom leaving main atom for example in NH4 N is main atom and there is 4 sorrounding atom then according to formula H equals SAhalfG-VE for -ve charge and -E for ve charge where H is hybridation SA is sorounding atom G is valence electron for main atom V is valency of all sorrounding atoms E is no of charge In NH4 H. What is the hybridization of NH4.
- When selecting a ball from your model kit to serve as the phosphorous atom from above how many holes should the ball have. The steric number can be found by adding the number of bonded atoms and then num. 5 a PFS b XeF6 c BH3 d CIFs e Brz 7.
Hybridization Of NH4 The hybridization of ammonium ion NH4 is sp3 because nitrogen atom in ammonium has only four bonding orbitals. It is an inorganic white crystalline salt that gets transparent on different wavelengths making it a suitable choice for optics. Structure of a covalent molecule we will distinguish between two kinds of electron pairs.
A BCl 3 b CH 2 F 2 c HCN d SCl 2 e PF 3 f HNO. 3 a CHCl3 b NH4 c H2CO 6. Draw the Lewis structure name the molecular geometry shape draw a three-dimensional sketch indicate the bond angle and state whether each of the following molecules is polar or nonpolar.
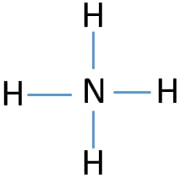
NH3 Lewis Structure Molecular Geometry Hybridization Bond Angle Shape. Write the Lewis structure. NH4 Lewis Structure Molecular Geometry and Hybridization NH3 is the chemical formula of Ammonia.
The molecule has a pungent smell. The valency of carbon is 4 and hence it can form 4 sigma bonds with four hydrogen atoms. - What is the molecular geometry around the central atom in CO2.
Calculate the number of sigma σ bonds.

Nh4 Lewis Structure Ammonium Ion Youtube

Lewis Structure Hybridization Nh4 Youtube

Methyl Radical Definition Formula Shape Bond Angle Hybridization Digital Kemistry In 2021 Chemistry Chemistry Lecture Chemistry Lessons

What Is A Functional Groups In Organic Chemistry Digital Kemistry Functional Group Organic Chemistry Chemistry
What Is The Hybridisation Of Nh4 Quora
What Is The Hybridisation Of Nh4 Quora

What Is The Difference Between Shell Sub Shell And Orbitals Structure Of Atom Atomic Structure Submarine Atom

Nh4 Molecular Geometry Shape And Bond Angles Youtube
What Is The Hybridisation Of Nh4 Quora

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Hybridization For Nh4 Description Of Hybrid Orbitals For Nitrogen Youtube
Nh4 Ammonium Ion Lewis Structure

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
Hybridization For Nh4 Description Of Hybrid Orbitals For Nitrogen Youtube
What Is The Nh4 Lewis Structure Quora

Nh4 Lewis Structure Molecular Geometry And Hybridization Techiescientist