Pbr3 Lewis Acid Or Base
Lewis acids accept electrons. Symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion.

N Heterocyclic Carbene Induced Reductive Coupling Of Phosphorus Tribromide Isolation Of A Bromine Bridged P P Bond And Its Subsequent Reactivity Chemical Science Rsc Publishing Doi 10 1039 C6sc02343f
My question is why is it favorable for PBr 3 to act as a Lewis acid ie.

Pbr3 lewis acid or base. List molecules Acid and Base. BBr_3 is a Lewis Acid. What type of bond is PBr3 and PBr3.
Is BBr3 a lewis acid or base. While Chlorine atoms have received the one needed. Ralph Pearson classified all Lewis acids and bases as hard and soft acids and bases.
BBr_3 is a Lewis Acid The reason it is a Lewis Acid is because it is an electron-pair acceptor. Phosphorus tribromide like PCl 3 and PF 3 has both properties of a Lewis base and a Lewis acid. Being short of the preferred octet BF 3 is a very good Lewis acid and reacts with many Lewis bases.
A fairly easy way to see this is to draw the Lewis dot structure of the molecule. In the Lewis structure for PBr3 there are a total of 26 valence electrons. A fairly easy way to see this is to draw the Lewis dot structure of the molecule.
Youll find that Boron has an incomplete octet. Lewis Acids and Bases. But in case of PF3 phosphorus be.
BBr_3 is a Lewis Acid The reason it is a Lewis Acid is because it is an electron-pair acceptor. A fairly easy way to see this is to draw the Lewis dot structure of the molecule. Lewis base gives out a lone pair of electronsN of NH3 has a lone pairSo it can act as Lewis base.
So a Lewis acid-base reaction is represented by the transfer of a pair of electrons from a base. Hence Br2 is considered to be a soft Lewis acid. Soft acids have large acceptor atoms of low positive charge high polarisability and low electronegativity.
PH3 Phosphine is lewis base What is an acid base neutral. Do not hesitate to hit that ORDER. Okay lets solve this problem.
A Lewis acid is a substance that accepts a pair of electrons to form a covalent bond. Accept electrons from the oxygen to form a bond when it already has a full octet and no formal charge. Youll find that Boron has an incomplete octet.
How to Draw the Lewis Dot Structure for ClBr3. Generally molecules that violate the octet rule act as Lewis Acids. The calculation for formal charge can be done using the formula given below-.
Ill tell you the Acid or Base list below. BBr_3 is a Lewis Acid The reason it is a Lewis Acid is because it is an electron-pair acceptor. Lewis bases donate electrons.
Its a weak base but has a lone pair of electrons. Youll find that Boron has an incomplete octet. But before going into reactions involving PCl3 it is very necessary to know about the structure hybridization and bonding of this liquid.
A fairly easy way to see this is to draw the Lewis dot structure of the molecule. Is Bbr3 A Lewis Acid Or Base. PBr3 is lewis Base.
Gilbert Lewis 18751946 proposed a third theory of acids and bases that is even more general than either the Arrhenius or Brønsted-Lowry theories. Hard acids have small acceptor atoms of low polarisability and. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
Generally molecules that violate the octet rule act as Lewis Acids. Generally molecules that violate the octet rule act as Lewis Acids. In PH3 phosphorus atom is attached to less electronegative hydrogen atoms the lone pair is readily available for donation therefore acts as Lewis base.
Generally molecules that violate the octet rule act as Lewis Acids. BBr_3 is a Lewis Acid. A fluoride ion is the Lewis base in this reaction donating one of its lone pairs.
Youll find that Boron has an incomplete octet. A fairly easy way to see this is to draw the Lewis dot structure of the molecule. A Lewis base is a substance that donates a pair of electrons to form a covalent bond.
In the following reaction each of two ammonia molecules Lewis bases donates a pair of electrons to a silver ion the Lewis acid. Br2 and Br are soft Lewis acids and Br- has properties in between soft bases and hard bases. Youll find that Boron has an incomplete octet.
An acid-base reaction in the Lewis sense involves formation of a coordinate covalent bond. According to the Lewis definition an acid is any species which can accept a lone pair of electrons and a base is any species which can donate a lone pair of electrons. Calculate the concentrations of PCl5g and PCl3g at equilibrium.
The reason it is a Lewis Acid is because it is an electron-pair acceptor. It would be nonpolar covalent because the electronegativity. Generally molecules that violate the octet rule act as Lewis Acids.

Phosphorus Tribromide Alchetron The Free Social Encyclopedia

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube

Substitution With Pbr3 Socl2 Video Lecture Chad S Prep

Is Pbr3 Polar Or Nonpolar Techiescientist

Pin By Sarah Hawkins On Chemistry Class Molecular Geometry Teaching Chemistry Chemistry Class

Why Does Phosphorus Tribromide Act As A Lewis Acid Electron Acceptor Chemistry Stack Exchange
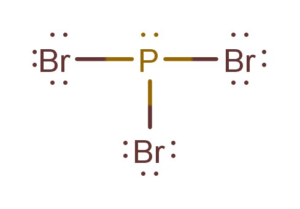
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity
Phosphorus Tribromide Br3p Chemspider

Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity
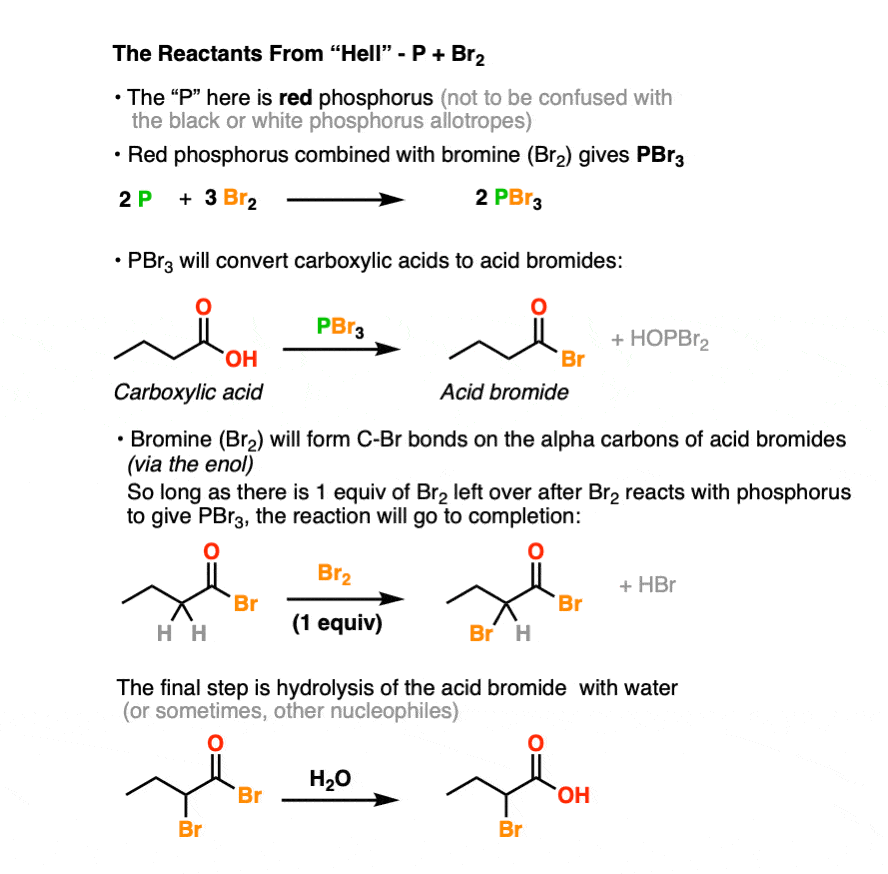
The Hell Volhard Zelinsky Reaction Master Organic Chemistry

Is Pbr3 A Lewis Acid Or Base Or Neutral
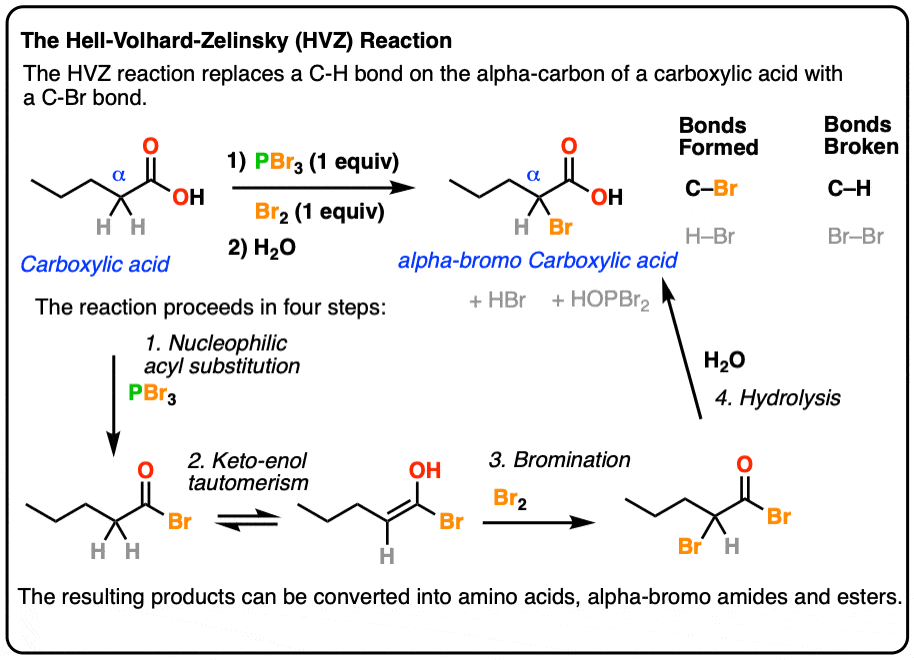
The Hell Volhard Zelinsky Reaction Master Organic Chemistry

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Phosphorus Tribromide Pbr3 Pubchem