Xef4 Lewis Structure Bond Angle
This is particularly explained by the valence bond theory as the two non-bonding electrons from 5p are promoted to the 5d orbital. Compound Lewis Structure Bond Angle s Molecular Shape sketch and name HNO HẠN 0 PF3 F-P-F XeF4 F-XeF H02 name shape at each H-O-0 CH2CH2 name shape at each H-CC H-C-H Resonance Structures Molecular Shape Bond Angle s Compound HCO - H-C-0 0-C-O Clso CIS-O C-SHI N20 N-NH.
Formula Lewis Structure Bond Angle S Molecular Shape Chegg Com
This compound written as TeF4 is 34 valence electrons not The person who asked you the question is looking for this.

Xef4 lewis structure bond angle. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. 19 Xef4 Molecular Geometry Bond Angle The Latest. Therefore there are 5 pairs.
The bond angles are therefore all 90 degreesXeF4 Lewis Structure - How to Draw the Lewis Structure for XeF4The question is nonsense. The Fluorine atoms are located at 90 degrees to each other resulting in the symmetric distribution of the electrons in the molecules plane. XeF4 Bond angles.
They must go on the Te. The geometry of the SCl4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the SCl4 geometrical shape in which the. Xef4 bond angles the bond angles of f xe f are 90 degrees and lone pairs have angles of 180 degrees.
SOLUTION a The Lewis structure of the SnCl3 ion looks like this. Since there are 4 electron groups around carbon the electron geometry is tetrahedral whose ideal bond angle is 1095. Transcribed Image Textfrom this Question.
The bond angles of F-Xe-F are 90 degrees and lone pairs have angles of 180 degrees. Xef4 has a square planar molecular shape so all the bond are in the equatorial position. In the XeF4 MO diagram it is quite clear that the structure of the compound is square planar.
Or we can say there is greater p-character in the hybrid orbital set which means that the bond angles are much closer to 90 degrees. The bonds are polar but the. Therefore XeF4 molecular geometry is square planar.
Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. The lone pairs lie on the opposite sides of the molecule basically at 180 from each other. And bond angle is 90 each.
Xef4 Shape Bond Angle. The two lone pairs are in the axial positions. Lewis Structure 3D Drawing E-F Hybr.
The Lewis structure for XeF4 has a total of 36 valence electrons. 4 bonds and 0 lone pairs. XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other.
For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Set your categories menu in Theme Settings - Header - Menu - Mobile menu categories. XeF 4 Molecular Geometry And Bond Angles.
The Lewis structure of CCl4 is. Therefore xef4 molecular geometry is square planar. Preparation of xef4 there are two methods is used for the preparation of xenon tetrafluoride.
90 degrees and 180 degrees. It has a distance of 195 A between the Xe and F. The SCl4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SCl4 molecule.
You have two left over. These bond angles contribute to the formation of square planar molecular geometry. The bond angles of F-Xe-F are 90 degrees and lone pairs have angles of 180 degrees.
So it converted octahedral to square planner. You should get four bonds to four Fs about the Te then 3 lone pairs about each F which gives you 32e so far. What is the bond angle of xef4 XeF4 has a square planar molecular shape so all the bond are in the equatorial position.
This means they are all on the same plane. This means they are all on the same plane. Draw the Lewis structure.
Molecular geometry polarity tutorial. 1100 Millecento Brickell 1100 South Miami Ave. The bond angles are roughly 90 degrees with some distortion due to repulsion by the lone pairs.
Which of the following compounds exhibit at least one bond angle the is. Sncl3 Lewis Structure What is the molecular geometry of sncl3. Thus the bond angle of CCl4 is 1095.
Xef4 shape and xef4 bond angle If you aluminate the loan pair then it gives square planner structure. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. Therefore this is an octahedral shape with 90 degree angles between each bond and lone pair of electrons.
Carbon is surrounded by 4 electron groups. Loan pair is repulsion.
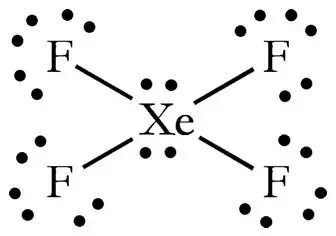
How Can The Lewis Structure For Xef4 Be Determined Quora

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube
What Is The Vsepr Structure Of Xef4 Quora

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
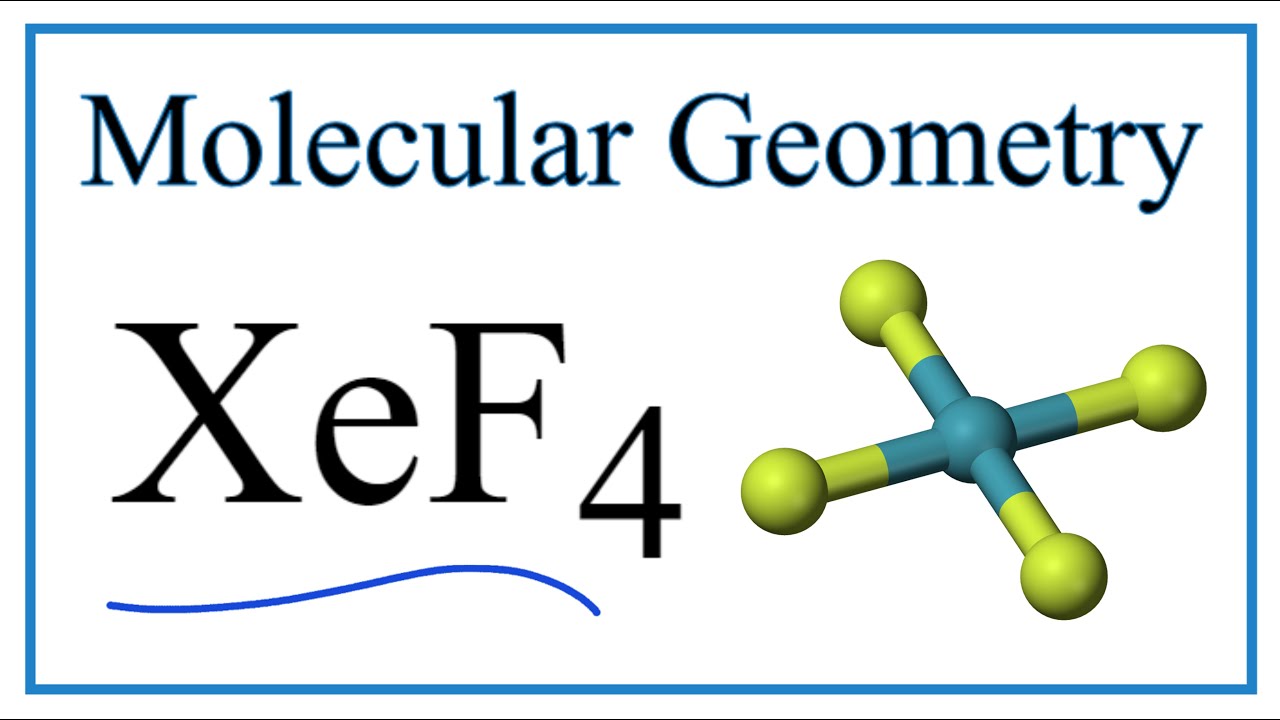
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube
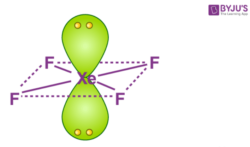
Hybridization Of Xef4 Hybridization Of Xe In Xenon Tetrafluoride
Why Is The Shape Of Xef4 Not Tetrahedral Quora

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Xef4 Lewis Structure And Molecular Geometry Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

Molecular Geometry Predicted By Vsepr Ppt Download
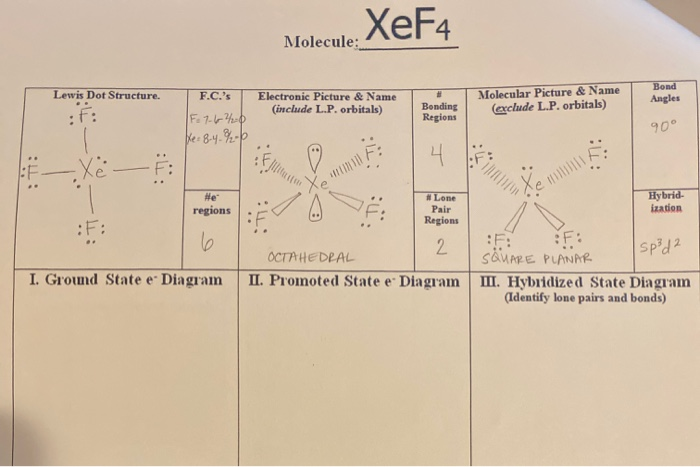
Xef4 Molecule Lewis Dot Structure F C S Electronic Chegg Com

Hybridization Of Xef4 Xenon Tetrafluorid Youtube
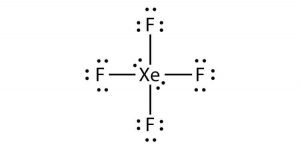
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
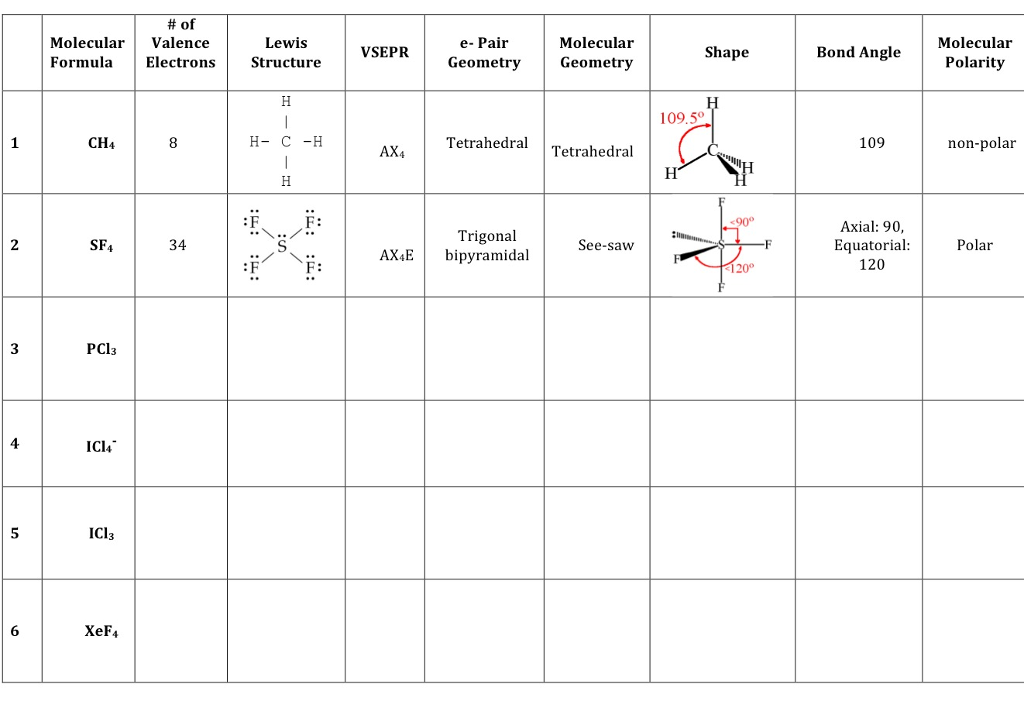
Of Molecular Valence Formula Electrons Ch4 Sf4 34 Chegg Com

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
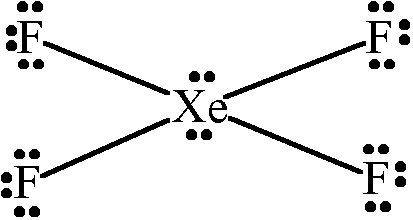
Which Of The Following Statments Concerning Xef4 Is Chegg Com
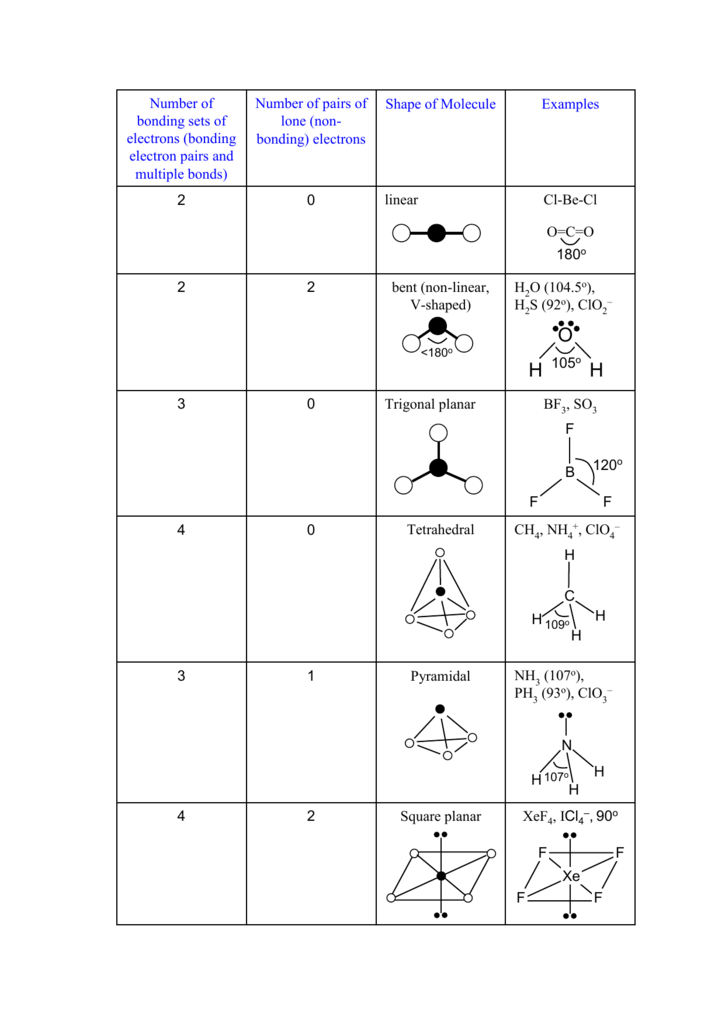
Xef4 Icl4 90o Square Planar 2 4 Nh 107o Ph 93o Clo

Xef4 Lewis Structure And Molecular Geometry Youtube