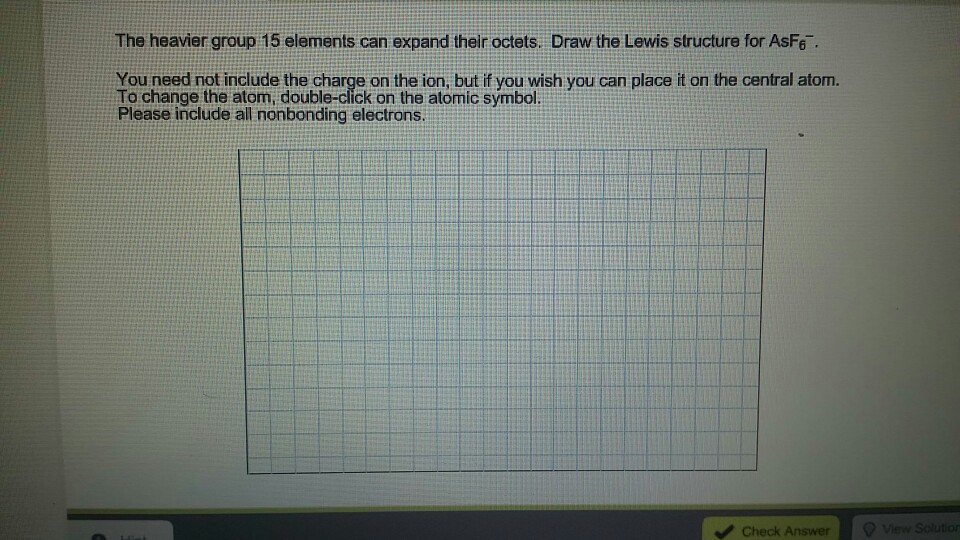
Asf6- Lewis Structure Formal Charge
Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. Include all lone pairs of electrons.

Exceptions To The Octet Rule Calango Free Online Courses
Draw Lewis Structure Of Co lewis structure sbr6 dot diagram draw o2 carbon hexabromide sulfur tetraiodide compounds ci4 lewis structure carbon monoxide oxygen dot bond formal wps formed three charge prenhall molecule bonds objects following atom electrons stable lewis structure molecular geometry draw hybridization dot carbon monoxide compound electrons valence study formula lewis.

Asf6- lewis structure formal charge. A stepbystep explanation of how to draw the krf4 lewis dot structure krypton tetrafluoride for structure calculate total number va. But now if you recalculate the formal charges for each of the atoms youll find that theyre all 0. Lewis structure draw snf6 ion resonance formal charges electrons pairs lone include structures needed solved possible.
Show the formal charges of all atoms in the correct structure. 3 Ways to Draw Lewis Dot Structures -. One last thing we do need to do though is add brackets around this to show that its an ion.
Formal charges are just that - a formality a method of electron book-keeping that is tied into the Lewis system for drawing the structures of organic compounds and ions. Photograph PF6 Lewis Structure. This is what we want.
Tang 05 formal charge lewis. This video answers the question. How to draw lewis structure for sif4.
And if you check the formal charges for each atom in the AsF6- Lewis structure youll see that they are 0. Images for Asf6 Lewis Structure How To Draw The Lewis Structure For. How to Draw the Lewis Structure for.
To calculate the formal charge on each atom in structure I and structure II find the number of valence electrons and subtract the number of nonbonding electrons and one-half the number of bonding electrons. Sif4 Lewis Structure How To Draw The Dot For Play Download. That gives us a -1 formal charge for the Oxygen atom.
Were still using the 32 valence electrons we had for the Cl3PO Lewis structure. In lewis structure of sulfate ion there should be charges on several atoms due to -2 charge. Photograph Exceptions to the Octet Rule.
To change the symbol of an atom double-click on the atom and enter the letter of the new atom. How to Draw the Lewis Structure for. Images for Asf6 Lewis Structure And Formal Charge.
How to Draw the Lewis Structure for. Later we will see how the concept of formal charge can help us to visualize how organic molecules react. For use periodic table find tota.
A stepbystep explanation of. How to Draw the Lewis Structure for SF6 - YouTube. Asf6 Lewis Structure And Formal Charge.
What we can do is take these two valence electrons on the Oxygen and form a double bond with the Phosphorus. How to Draw the Lewis Structure for. And this is the Lewis structure for AsF6- Hexafluoroarsinate ion.
Photograph AsF6- Lewis Structure. The formal charges closer to zero mean a more plausible or likely Lewis structure. There are a total of 48 valence electrons in the Lewis structure for SF6.
Usually you circle the charge so its clear This can also help you tell which Lewis structures are good. Draw Lewis structures for each of the following species. How to Draw the Lewis Structure for.
Draw the molecule by placing atoms on the grid and connecting them with bonds. For asf6 structure calculate total number valence electrons as. How to Draw the Lewis Structure for.
Part A Use formal charge to identify the better Lewis structure betwwen the following. Show the formal charges of all nonhydrogen atoms or use square brackets to denote the overall charge. Hexafluoroarsenic1- AsF6- CID 23515 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
When you write Lewis structures you should include formal charges next to each atom with a formal charge that isnt 0. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom. Usually negative formal charges should be on atoms that pull electrons strongly like O or F elements from the top right of the periodic table that have high ionization energies and high electron affinities.
Photograph Cl3PO Lewis Structure. Sulfate ion sulfate ion SO 4 2- Sulfate ion is one of the oxyanion of sulfur. How to Draw the Lewis Structure for.
How to Draw the Lewis Structure for. Draw Lewis structure for. Photograph BrO2- with Formal Charges - YouTube.
For the SF6 Lewis structure you should take formal charges into account to find the best Lewis structure.

Predict The Molecular Geometry Of Asf6 Arsenic Hexafluoride Youtube
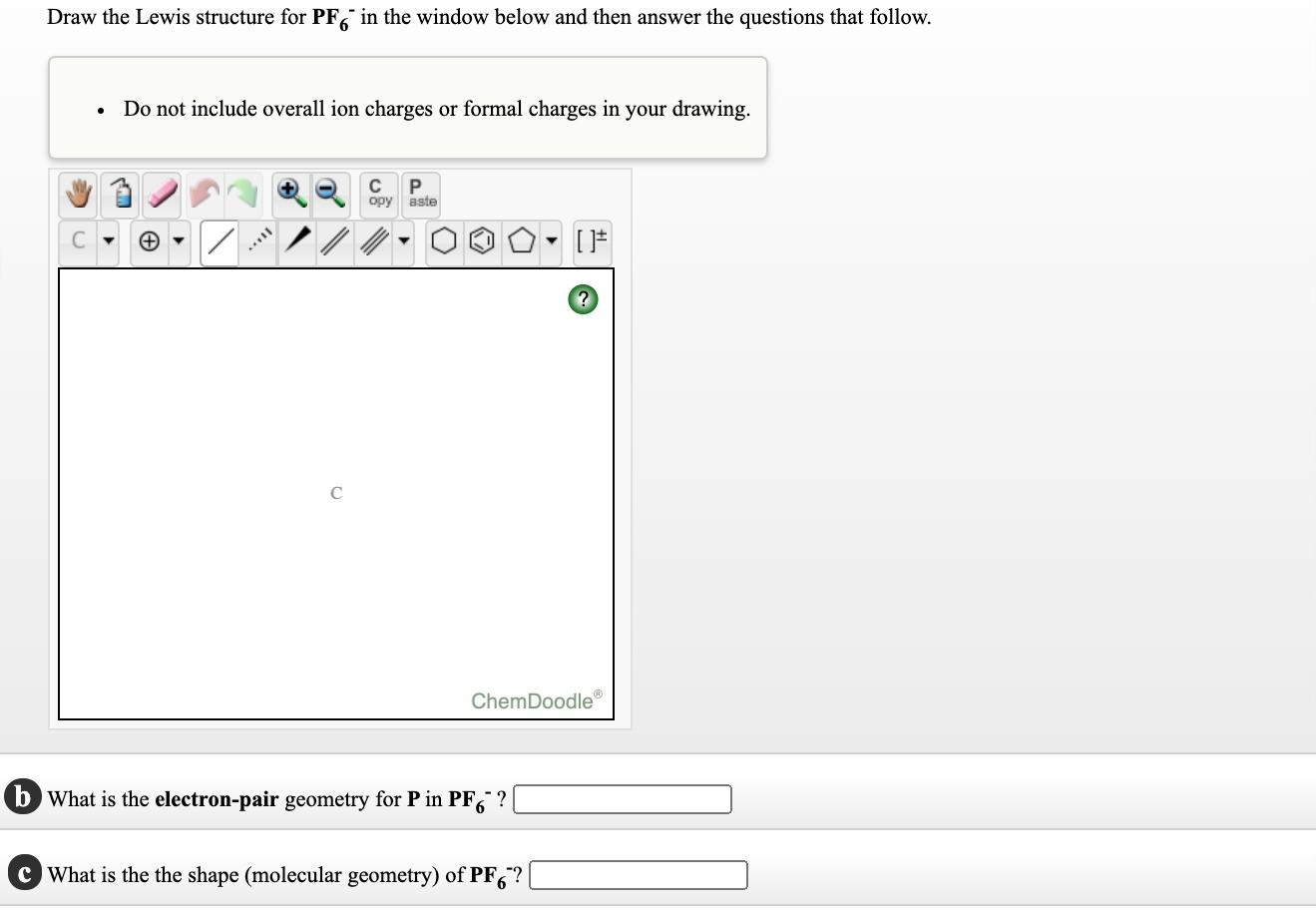
Draw The Lewis Structure For Pf6 In The Window Below Chegg Com
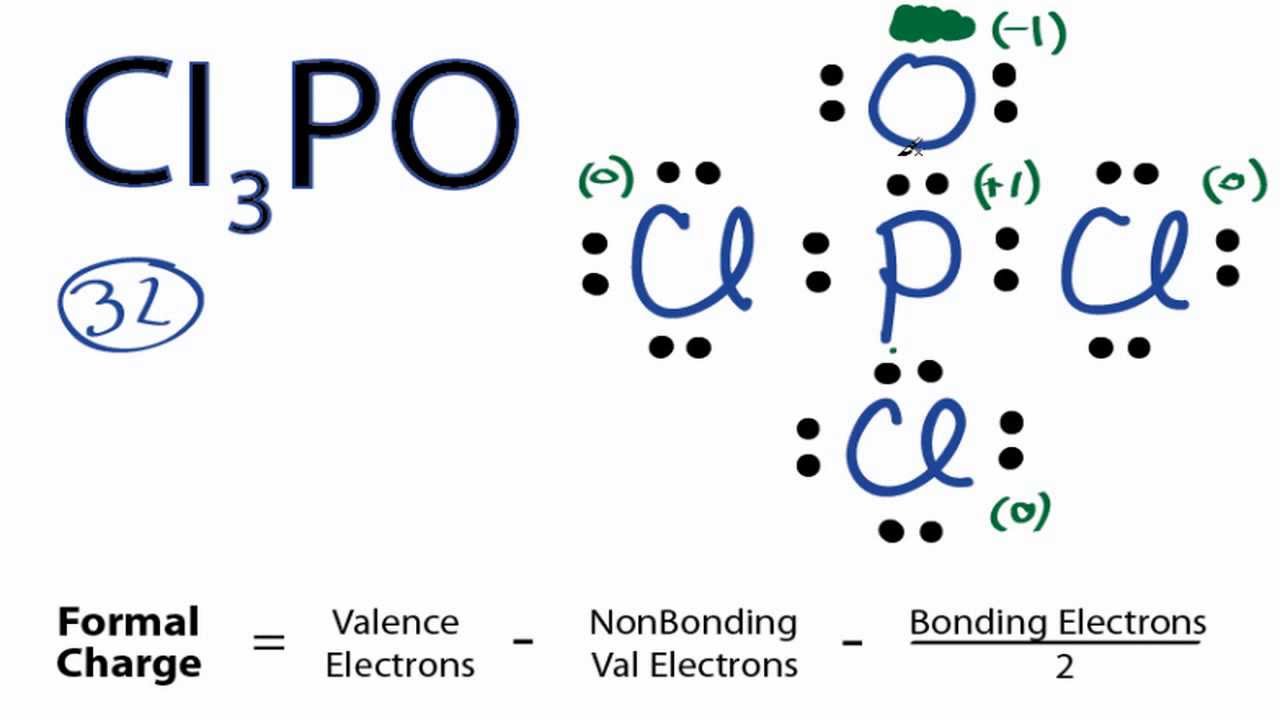
Cl3po Lewis Structure How To Draw The Lewis Structure For Cl3po Youtube
The Heavier Group 15 Element Can Expand Their Chegg Com

What Is The Lewis Structure For Seobr 2 Clutch Prep
Hexafluoroarsenate Asf6 Chemspider

How To Draw The Lewis Structure For Clf2 Youtube

Draw Lewis Structures For Each Of The Following Species Show The Formal Charges Of All Atoms In The Correct Structure Include All Lone Pairs Of Electrons A Asf6 B Cl3po C If5
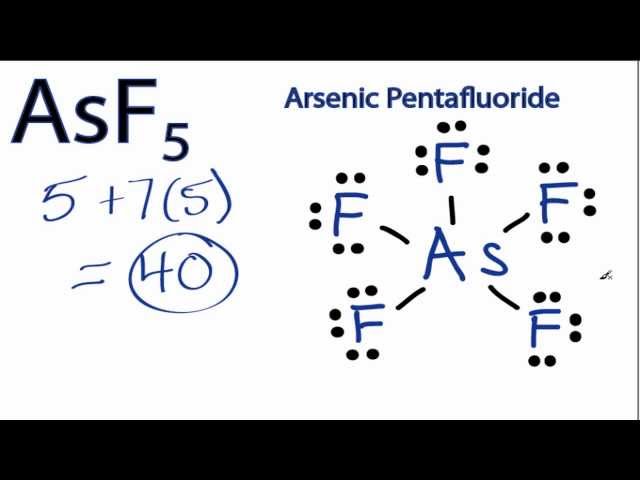
Asf5 Lewis Structure How To Draw The Lewis Structure For Asf5 Youtube

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube
Dioxygenyl Hexafluoroarsenate Asf6o2 Pubchem

Formal Charge The Charge A Bonded Atom Would

Exceptions To The Octet Rule Calango Free Online Courses

Equilibrium Structures Of The Asf 6 Superhalogen Anion And The Download Scientific Diagram

Clf5 Lewis Structure How To Draw The Lewis Structure For Clf5 Chlorine Pentafluoride Youtube
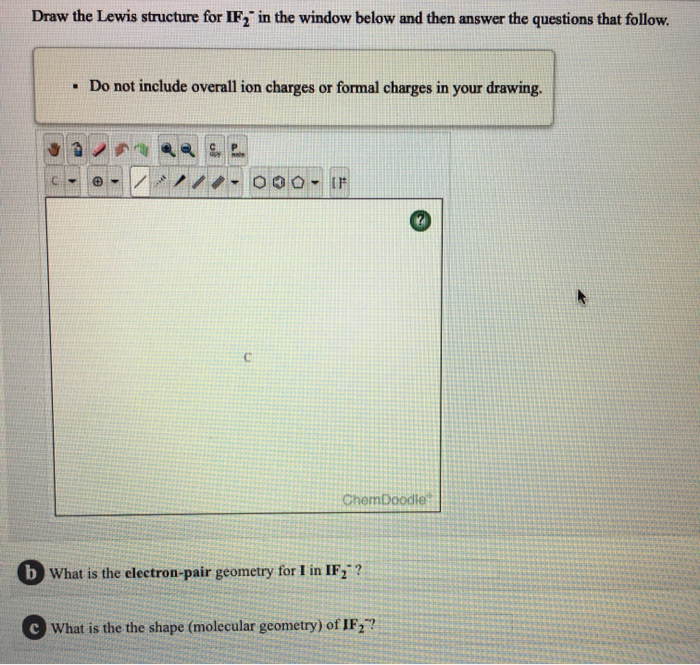
Draw The Lewis Structure For If In The Window Below Chegg Com

Asf6 Lewis Structure How To Draw The Lewis Structure For Arsenic Hexafluoride Ion Youtube
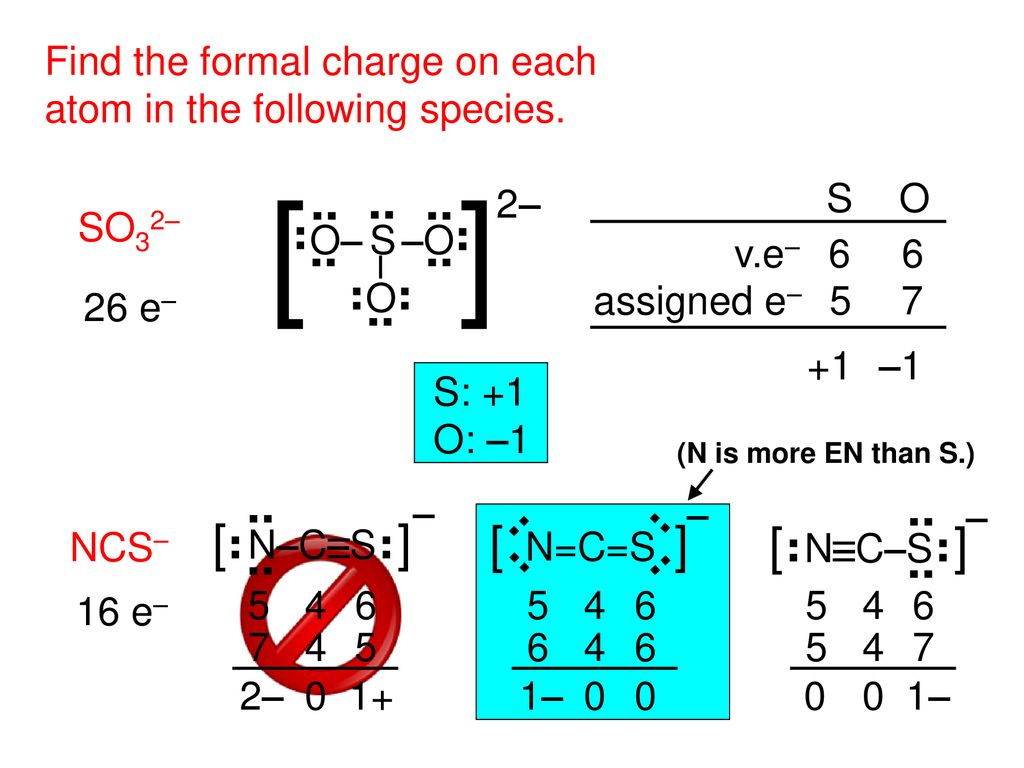
Formal Charge The Charge A Bonded Atom Would Have Ppt Download