Brf5 Lewis Structure Valence Electrons
3 however is possible. 8 electrons of the total valence.
How To Determine The Lewis Structure For Pcl5 Quora
Second place the valence electron on the iodine and hydrogen atoms.

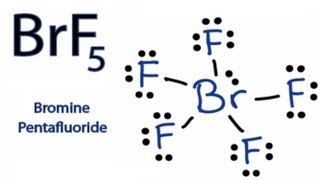
Brf5 lewis structure valence electrons. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. BrF5 is predominantly used as a fluorinating agent to produce fluorocarbons and as an oxidizer in rocket propellant systems. So from the total of 42 valence electrons available for the BrF5 lewis structure we employed 10 electrons for 5 single Br-F bonds in the BrF5 molecule.
Because each bromine atom is connected to a fluorine atom by five single Br-F bonds each connection contains two electrons. Metal chlorides bromides and iodides are converted to fluorides by treatment with BrF5. Total outermost valence shell electrons available for HBr Lewis structure dot structure 711 8 valence electrons in HBr.
In this post we discussed the method to construct the CH3I Lewis structure. Now we will put the 28 valence electrons around the atoms to achieve octet fulfillment. Calculation of total valence electron of HBr molecule.
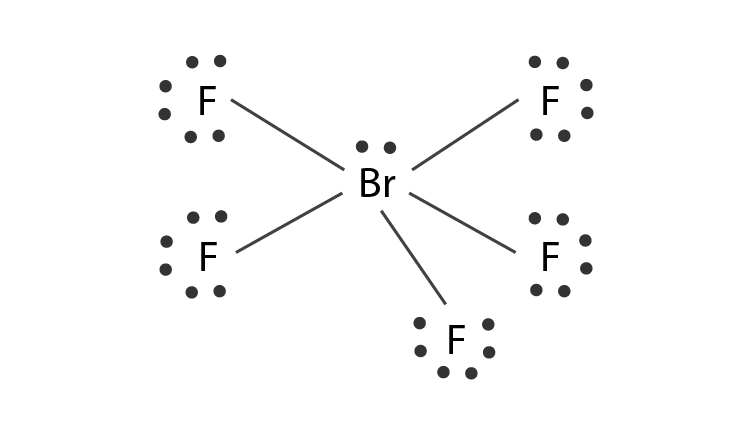
The Lewis structure of a compound represents a schematic arrangement of all the atoms present in the compound. We have achieved our octet configuration for every atom here. Brf5 structure vsepr chemistry electron brf valence pair repulsion theory shell.
So 4 plus 4. Step 4There will be a single bond formation with bromine and each of the fluorine atoms. First the valence electrons are placed around the carbon atom.
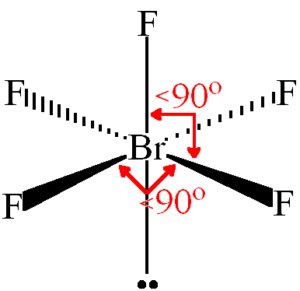
7Br 35F 42 Valence Electrons. Based on VSEPR theory predict the electron-pair and molecular geometries for this molecule. Molecular geometry trigonal bipyramidal Oc electron-pair geometry octahedral.
Is XeF2 a Lewis structure. Each one of these 3 electrons has the potential to form one single bond with another compound. The molecular geometry of BrF5 is square pyramidal with.
In the Lewis structure for BrF 5 there are a total of 42 valence electrons. Summary The total valence electron available for the BrF5 lewis structure is 42. Molecular geometry square pyramidal O d electron.
NO3- SO42- BrF5 C2H2 XeCl4. In the periodic table silicon group 4 4 valence electrons hydrogen group 1 1 valence electron but we have four. Brf5 Lewis Structure Vsepr Can Lewis structures predict the shape of a molecule VSEPR Method by G Dupuis and N Berland The Shapes Of Molecules Predicting the Geometry of Molecules and Polyatomic Ions.
It is important to understand that chemistry is full of exceptions where Sulfur bromine can also expand their octet to accommodate a greater number of valence electrons. Bromine pentafluoride BrF5 is a liquid with a sharp penetrating odor. The hybridization of BrF5 is Sp³d².
BF5 is Boron Pentafluoride and remember that Boron Pentafluoride has 3 electrons on its outermost shell P-shell. Possible Formula Number of Lewis Structure Valence electrons Formal charge on central atom resonance structure Yes or No H2 PF3 N2 C2H4 CO2 AIC13 PO43- 13. Once we know how many valence electrons there are in BrF5 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
But as per the octet rule the outermost shell can have a maximum of 8 eight valence electrons but bromine is having 12 as per the Lewis structure drawn for BrF5. For the BrF 5 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. Therefore the total number of valence electrons in BrF5 is given by.
But if we count the total number it counts up. There are a total of 22 valence electrons in the Lewis structure for XeF2. A step-by-step explanation of how to draw the BrF2 Lewis Dot StructureFor the BrF2 structure use the periodic table to find the total number of valence el.
There are still 32 valence electrons left in the BrF5 molecule. BrF5 or bromine pentafluoride has a square pyramidal structure as in the first figure. 7 x 5 35 Valence Electrons.
Bromine pentafluoride is polar in nature. Lets do the Lewis structure for SiH4. Put Yes in the middle hydrogen always comes out.
A electron-pair geometry octahedral. Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of HBr. Note that in the Lewis structure for BrF5 Bromine B is in Period Four on the periodic table.
Br and the three F atoms each have eight electrons surrounding them as valence electrons. Molecular geometry square planar Ob electron-pair geometry trigonal bipyramidal. The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral.
In the BrF 5 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure. The Lewis structure of BrF5 is shown below. For the BrF5 Lewis structure the total number of valence electrons found on the periodic table is 42.
This means it can hold more than eight valence electrons. The problem here is that it cannot be found to 5 Fluorine atoms. What is the shape of BrF5.
But on the other hand BrF5 does have a dipole moment due to the asymmetric structure as shown earlier in the figures. Therefore the five Fluorine atoms present contribute.

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Make A Sketch Of Brf5 Clutch Prep
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Lewis Structure Of Brf5 Biochemhelp
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist