Brf5 Lewis Structure
In BrF5 lewis structure bromine is the central atom and it is connected with 5 single bonds to the fluorine atoms. Lewis dot structure of BrF 5.
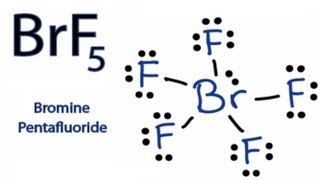
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Bromine pentafluoride BrF5 is a liquid with a sharp penetrating odor.

Brf5 lewis structure. In the BrF5 lewis structure bromine is the central atom and it is connected with 5 single bonds Br-F to the fluorine atoms. Bromine pentafluoride chemical formula is BrF5. It is a strong fluorination reagent.
Put Yes in the middle hydrogen always comes out. For the BrF 5 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. It means it already sharing 10 electrons with the help of 5 single bonds.
Bromine is the least electronegative well put that in the center and then well put 5 Fluorines around the outside. Lets do the Lewis structure for SiH4. First the valence electrons are placed around the carbon atom.
What is the Lewis structure of SiH4. Lone pairs are found in one of the hybrid orbitals. In the periodic table silicon group 4 4 valence electrons hydrogen group 1 1 valence electron but we have four.
Second place the valence electron on the iodine and hydrogen atoms. Step-by-step video of how to get from the formula BrF5 to its Lewis structure and geometry. Each one of these 3 electrons has the potential to form one single bond with another compound.
It can be observed from the BrF 5 Lewis structure that there are five Fluorine atoms surrounding the central Bromine atom. For the molecule BrF5 draw the Lewis structure arrangement geormetryshape and hybridization for non-hydrogen atoms. Laser ablation of solid silicates in the presence of bromine pentafluoride releases O2 for subsequent analysis.
Note that in the Lewis structure for BrF5 Bromine B is in Period Four on the periodic table. Bromine Pentafluoride comprises 5 Fluorine atoms all pulled together by the central Bromine atom. BrF5 Lewis Structure Molecular Geometry Hybridization and Polarity.
The steric number is an important term here which we need to find out for any VSEPR calculation. It means it already sharing 10 electrons with the help of 5 single bonds. Bromine pentafluoride BrF5 is an interhalogen compound and a fluoride of bromine.
In this post we discussed the method to construct the CH3I Lewis structure. In the liquid state it is a colorless fuming compound having a pungent odor. In the BrF 5 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure.
The problem here is that it cannot be found to 5 Fluorine atoms. BrF5 is predominantly used as a fluorinating agent to produce fluorocarbons and as an oxidizer in rocket propellant systems. Once we know the number of valence electrons in the BrF5 so after that its easy to distribute them throughout the nuclear atom to achieve the goal of an outer shell of every atom.
You are asking for the Lewis Structure of a compound that cannot exist. For human beings bromine pentafluoride is highly toxic and must not be inhaled at any cost as it is. Bromine Pentafluoride or BrF5 is a fluoride of bromine and an interhalogen compound which means it is made up of only halogen atoms.
Who are the experts. So bromine is obeying the rule of the octet as it has more than 8 electrons around it. Once we know how many valence electrons there are in BrF5 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
So 4 plus 4. What is the shape of BrF5. In BrF 5 one 4s three 4p and two 4d orbitals take part in hybridization.
To determine the hybridization we take a look at the Lewis structure of the BrF 5 molecule. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. We review their content and use.
The central atom bromine forms 5 sigma bonds with fluorine atoms. In the BrF5 lewis structure the total number of electrons is 42 that is found in the periodic table. BrF5 finds use in oxygen isotope analysis.
For BrF5 we have a total of 42 valence electrons. 8 electrons of the total valence. Drawing BrF5 Lewis Structure is very easy to by using the following method.
Metal chlorides bromides and iodides are converted to fluorides by treatment with BrF5. We have two lone pairs on the Bromine atom an exception to the octet rule. BF5 is Boron Pentafluoride and remember that Boron Pentafluoride has 3 electrons on its outermost shell P-shell.
So bromine is obeying the rule of the octet as it has more than 8 electrons around it. There is also a lone pair attached to the Bromine atom. Experts are tested by Chegg as specialists in their subject area.
It has also been tested as an oxidizer in liquid rocket propellants and is used as a fluorinating agent in the processing of uranium. 3 however is possible. This is the BrF5 Lewis structure.
Br is the central atom. For the BrF5 Lewis structure the total number of valence electrons found on the periodic table is 42. We have three Fluorine atoms surrounding the central Br atom therefore three bond pairs.
BrF 5 Molecular Geometry And Bond Angles. Here in this post we described. Let us have a look at the Lewis Structure again.
Use information from step 4 and 5 to draw the lewis structure. Calculate the total valence electrons in BF 3 molecule. Alternatively a dot method can be used to draw the lewis structure of BF 3.
Well draw single bonds between the atoms for a total of 5 single bonds so 10 valence electrons. In the Lewis structure for BrF 5 there are a total of 42 valence electrons.

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Would The Lone Pair Be In The Equatorial Plane Or The Axial Plane For Bromine Pentafluoride Chemistry Stack Exchange

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Make A Sketch Of Brf5 Clutch Prep

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
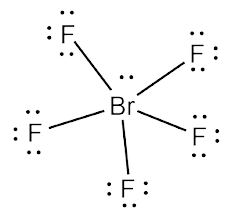
Is Brf5 Polar Or Nonpolar Bromine Pentafluoride Polarity Explained
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube
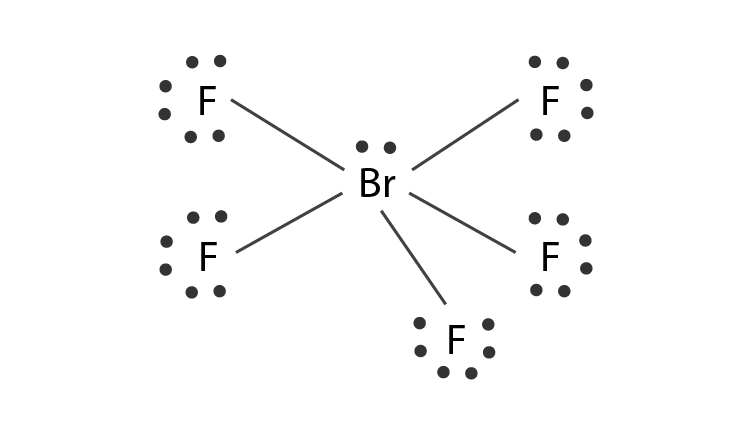
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
