Consider The Lewis Structure Of Scl2 Molecule. What Is The Formal Charge Of Sulfur
Key Points To Consider When Drawing The SCl4 Structure. A Does the Lewis structure depict a neutral molecule or an ion.

How To Draw Sf4 Lewis Structure Science Education And Tutorials
The formal charge on an atom is calculated as the number of valence electrons owned by the isolated atom minus the number of valence electrons owned by the bound atom in the molecule.

Consider the lewis structure of scl2 molecule. what is the formal charge of sulfur. Formal charge number of valence electrons owned by the isolated atom - number of valence electrons owned by the bound atom. That means that that means that its former charge is minus one. A three-step approach for drawing the SCl4 Lewis structure can be used.
Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. Resonance occurs in cases where two or more Lewis structures. To design the best Lewis structure you also need to calculate the formal charge of every atom too.
Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. Already have an account. If it is an ion what is the charge on the ion.
The product contains about 72 - 80 SCl2 residual S2Cl2 and Cl2. BO3 H2co XeF4 SCl6 Lewis Structure Molecular Geometry Hybridization Of Central Atom Bond Angle Of Central Atom Number Of σ Bonds Number Of π Bonds. Heartburn is the result of abdomen acid actually burning its way by means of your esophagus.
You know that both the Sulphur and Oxygen has six valence electrons each. Announcing Numerades 26M Series A led by IDG Capital. Consider the Lewis structure shown below.
D How many electrons are in the pi system of the species. The first step is to sketch the Lewis structure of the SCl4 molecule to add valence electron around the sulfur atom. Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70.
There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent. Log in Sam L. In a Lewis structure formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom.
This painful burning feeling happens within your chest when you take in a huge meal or if you lie down just after 1. Consider the Lewis structure shown below. This is in contrast to the electronic geometry which describes the shape of all electron regions.
C Are there multiple equivalent resonance structures for the species. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. Relevant to consider the lewis structure for the nitric acid molecule hno3 and select the false statement Heartburn is the acid reflux symptom many people understand.
Sulfur is the central atom in the Lewis structure of SCl2 has a steric number equal to 4 hence electron geometry of SCl2 is tetrahedral. Here we have two Oxygen atoms so a total number of valence electrons will be eighteen. The sulfur difluoride chemical formula is SF2.
A Does the Lewis structure depict a neutral molecule or anion. In b the sulfur atom has a formal charge of 0. SF 2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms.
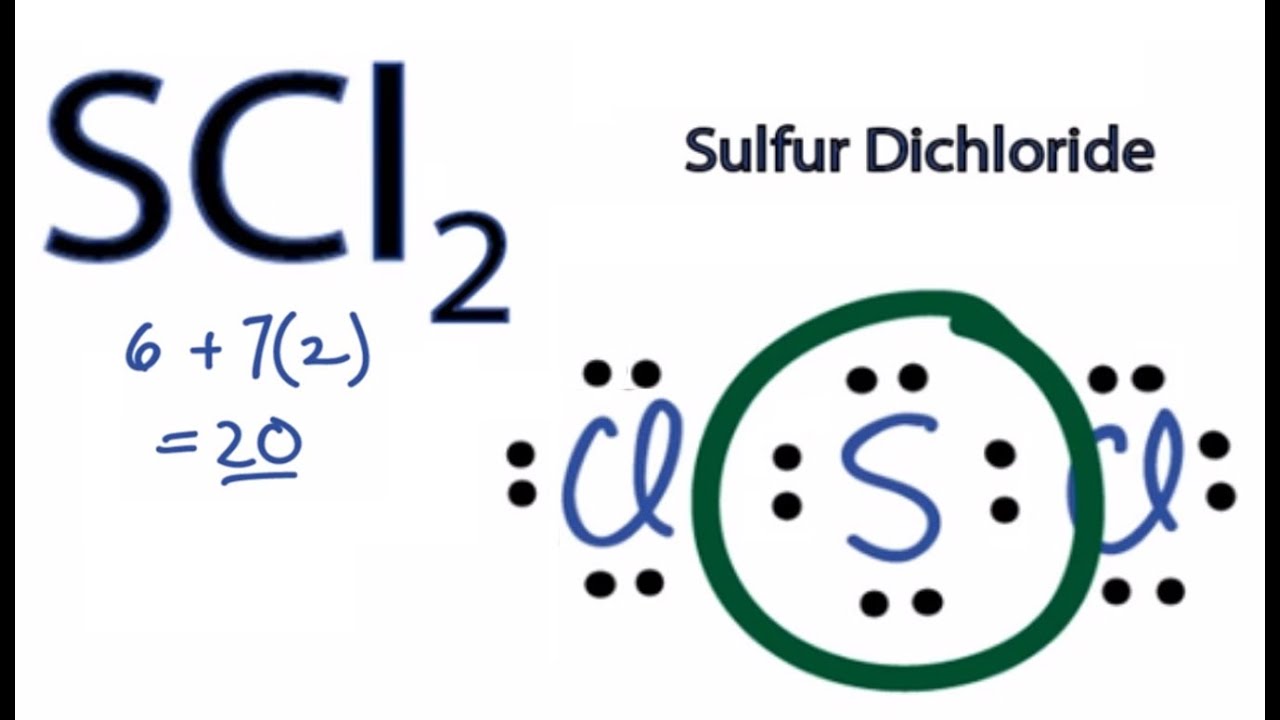
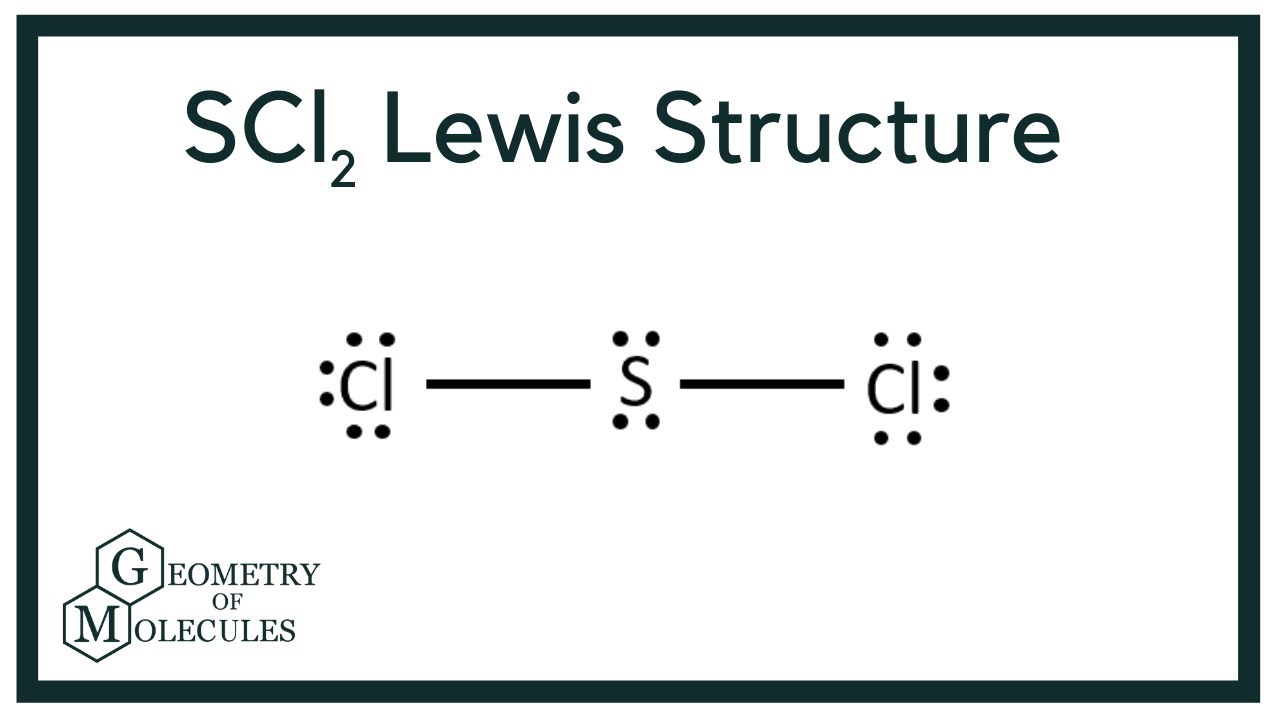
The formal charge on the sulfur atom is therefore 6 - 6 22 1. I Draw the. Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center.
Drawing SF2 Lewis Structure is very easy to by using the following method. Determining formal charge on an atom. The molecular geometry of a molecule describes the three-dimensional shape of just the atoms.
Stabilized pure sulfur dichloride should contain minimum 98 SCl2. Hence the molecular geometry of SCl2 is bent. To determine the molecular geometry of a molecule one must first determine the electronic geometry by drawing the Lewis structure.
For the SCl2 Lewis structure we have a total of 20 valence electrons. Xenon can be reacted with fluorine to form xenon tetrafluoride XeF4. The molecular geometry of SCl2 simply determined by the coordination number which is equal to 2.
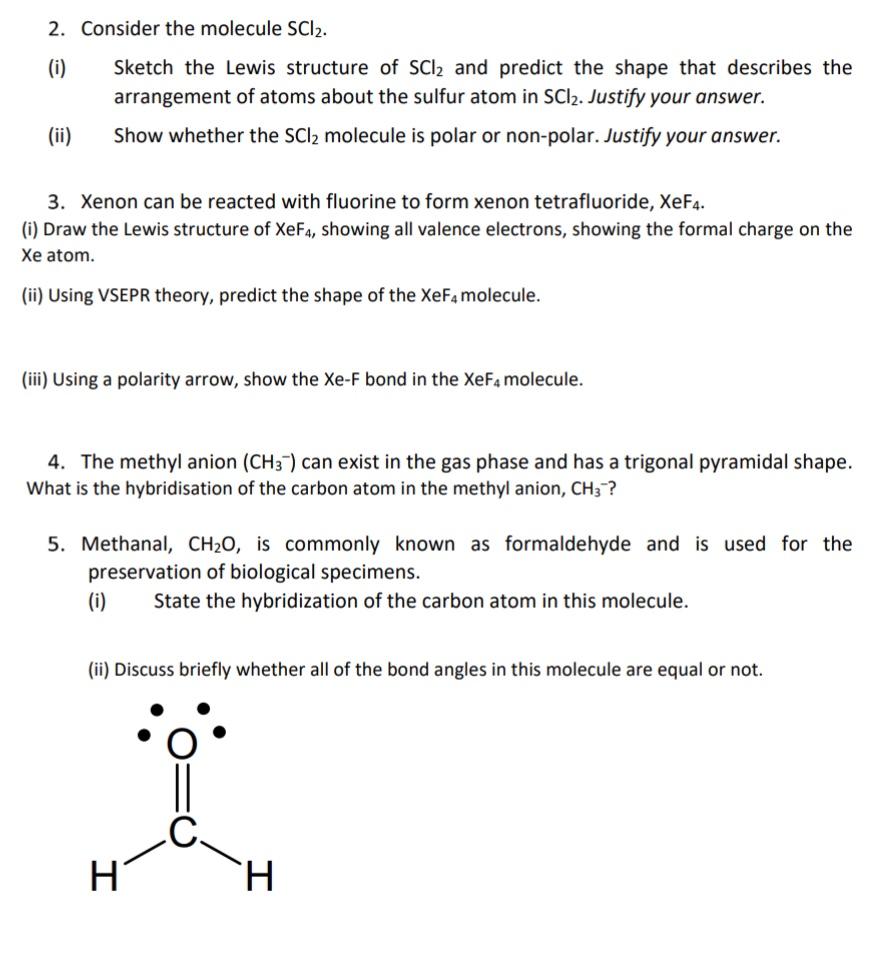
Lewis structure of SO 42- There are two SO bonds and two S-O bonds in sulfate ion lewis structure. To create the Lewis structure of SO2 you need to arrange the eight valence electrons on the Sulphur. I Sketch the Lewis structure of SCl2 and predict the shape that describes the arrangement of atoms about the sulfur atom in SCI2.
There are no lone pairs in the last shell of sulfur atom. Read how Numerade will revolutionize STEM Learning. The second step is to valence electron to the four chlorine atoms and the final step is to combine the step1 and step2 to get the SCl4 Lewis Structure.
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. A structure in which the formal charges are as close to zero as possible is preferred. Consider the molecule SCl2.
Does this depict a neutral Adam or Anne and I And the answer is as you see here and and and I because when oxygen is only forming one bond that Monash is only forming one bond. Continuing with sulfur we observe that in a the sulfur atom shares one bonding pair and has three lone pairs and has a total of six valence electrons. Lewis structure of the molecule What is the formal charge on carbon.
Molecular geometry of IF 2-1 and lewis structure. Six Chapman Chemistry Central Science says the Louis structure of the molecule depicted in your textbook. B What hybridization is exhibited by each of the carbon atoms.
Problem An element has electronic configuration 1 s2 View Full Video. Here in this post we described step. If it is an ion what is the charge on the ion.
The Sulphur atom has. Show whether the SCl2 molecule is polar or non-polar.

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Best Overview Is Cs2 Polar Or Nonpolar Science Education And Tutorials

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization
2 Consider The Molecule Scl2 I Sketch The Lewis Chegg Com

H2s Molecular Geometry Science Education And Tutorials
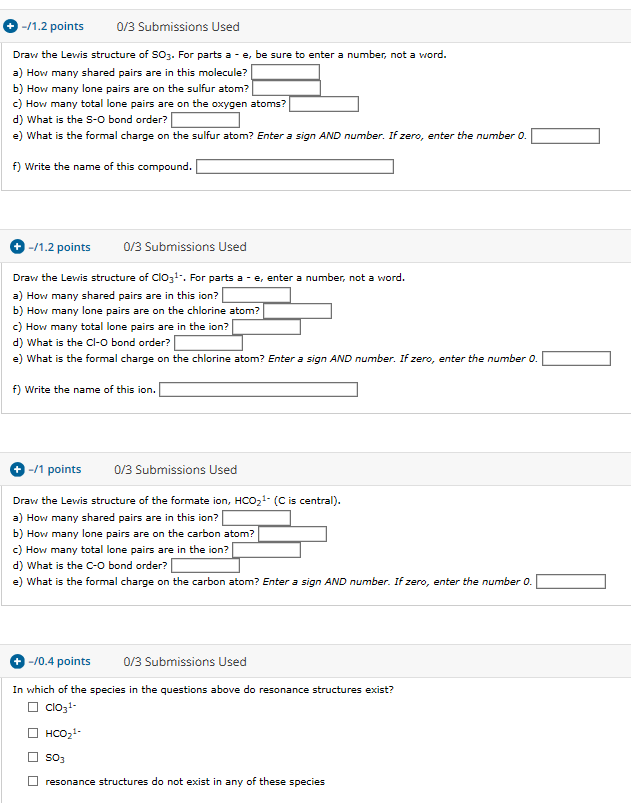
1 2 Points 0 3 Submissions Used For This And All Chegg Com

How To Draw Sf4 Lewis Structure Science Education And Tutorials
Scl2 Lewis Structure Sulfur Dichloride Youtube
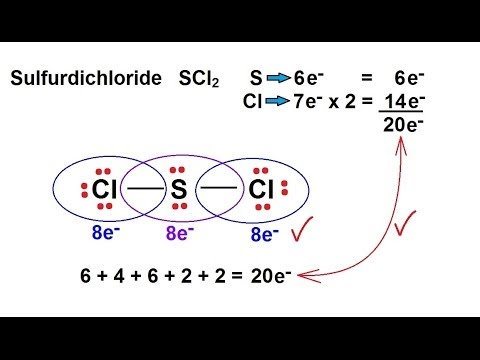
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
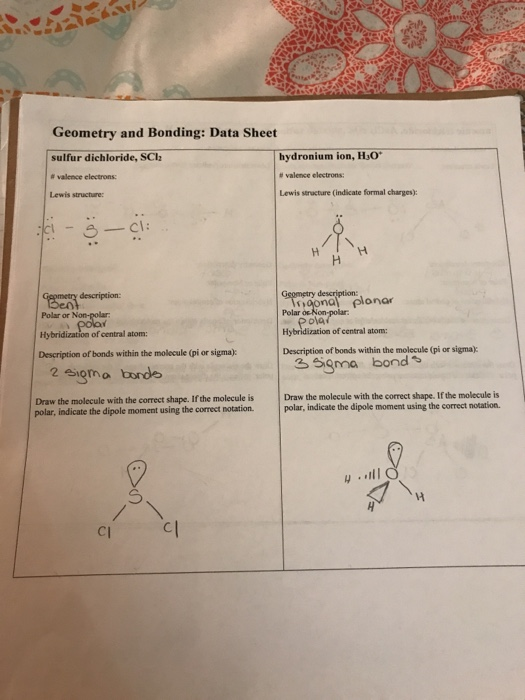
Geometry And Bonding Data Sheet Sulfur Dichloride Chegg Com

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Scl2 Lewis Structure Sulfur Dichloride Youtube

Formal Charges For So2 Sulfur Dioxide Correct Youtube

How To Draw Sf4 Lewis Structure Science Education And Tutorials

Best Overview Is So2 Polar Or Nonpolar Science Education And Tutorials

Socl2 Lewis Structure How To Draw The Lewis Structure For Socl2 Youtube