Correct Lewis Structure For C2h4
In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells. 2 See answers SimontheSalmon SimontheSalmon Remember this when trying to find a valid lewis structure every single atom must have a total of 8 shared electrons full octet.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
It has only 10e-instead of 12.
Correct lewis structure for c2h4. The clouds are grey and ground is wet. There are two triangles overlapping each other as we can see in the diagram. Central with 3 almost like a triangle.
There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell. Drawing the Lewis Structure for C2H For C2H4 you have a total of 12 total valence electrons. Subtract step 3 number from step 1.
Which is the correct Lewis structure for N 2 H 2. 6 rows Drawing the Lewis Structure for C 2 H 4. Which is the correct lewis structure for ethylene c2h4.
Physics 21062019 1700 lulustar13. Output voltage is assumed to be normally distributed with standard deviation 025 v and the manufacturer. Arrange hydrogen and oxygen on the sides.
Calculate the total valence electrons in the molecule. Which of the following is the correct lewis structure for C2H4. Use information from step 4 and 5 to draw the lewis structures.
Н 1 САН Н. Structure C has 14 2 extra electrons. Lewis dot structure of C 2 H 4.
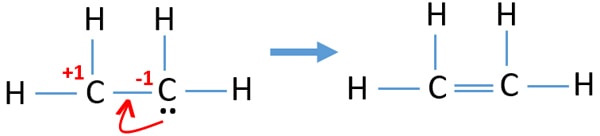
Since I only have 4 hydrogens to work with two on each carbon I assumed a double bond in the middle. Correct lewis structure for C2H4. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. A step-by-step explanation of how to draw the HSO4- Lewis Dot Structure Bisulfate ion or Hydrogen sulfate ionWhen we have an H or H2 in front of a polya. Which of the following covalent molecules contains polar bonds.
Which of the following has the highest electronegativity. нес-н т Н Н b. Lewis structure A is the correct answer.
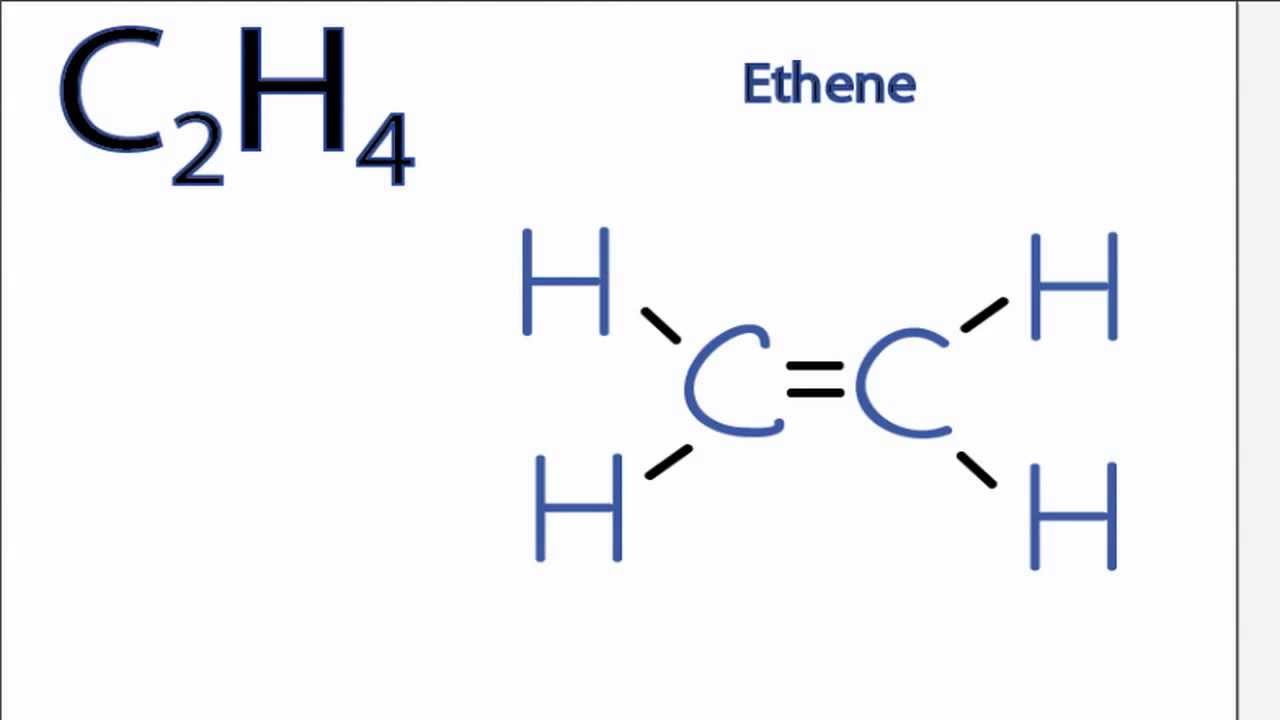
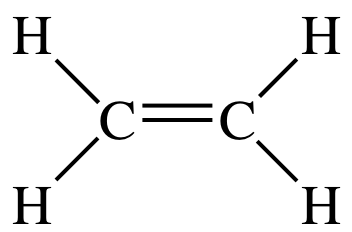
Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond. н-сон Н Н HT Н С. Which is the correct Lewis structure for ethylene C2H4.
Each of the N atoms satisfy the octet requirement and the H atoms follow the duet rule. 1 Show answers Another question on Chemistry. Matches the chemical name of each oxide of phosphorus to its chemical formula.
Which shape is the following molecule. Amanufacturer is interested in the output voltage of a power supply used in a pc. The key to understanding how to distribute the valence electrons is to.
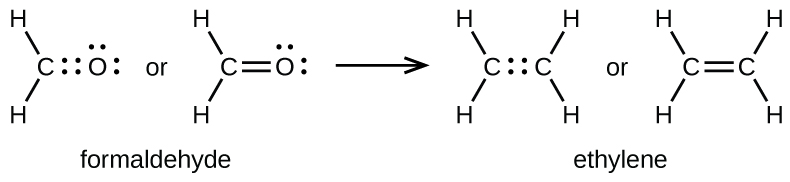
Alternatively a dot method can be used to draw the lewis structure. The following three structures are possible for C 2 H 4 O. 18-14 4e-2 lone pair.
Two carbon atoms go in the center side by side. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val. This means that the carbon atoms share 4 electrons.
2 Get Other questions on the subject. 2C 4 2 8. If the electronegativity difference between A and B is 08 what type of bond is formed between the two elements.
Electron Dot Structure for ethane C2H4. For C 2 H 4 you have a total of 12 total valence. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
Which of the following is the correct Lewis structure for CH4. Therefore there cannot be more than one stable resonance structure for C 2 H 4. Which of the following is the correct Lewis structure for C2H4.
Which is the correct Lewis structure for ethylene C2H4. Structure B is electron deficient. Use information from step 4 and 5 to draw the lewis structure.
It has a total of 2 x 5e- 2 x 1e- 12e-. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. An _____ is an atom with a positive charge.
Sothe total number of CH2CH2 valence electrons is twelve 8 412 These 12 valence electrons have two tasks at the the same timeThey connect all the atoms and satisfy the duet rule of hygrogens and octet rule of two carbon atoms.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Is C2h4 Polar Or Nonpolar Youtube
Lewis Symbols And Structures Chemistry 2e

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Lewis Structure For The C2h4 Ske Clutch Prep

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Top Of Page Periodic Table Andover S Chem 300 Accelerated Honors Chemistry Table Of Contents Chapter 9 Chemical Bonding And Nomenclature Section 9 1 Relationship Between Groups And Number Of Valence Electrons Section 9 2 Electronegativity
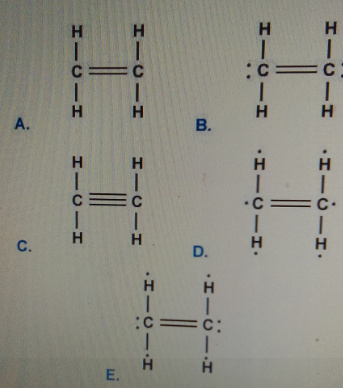
Which Is The Correct Lewis Structure For Ethylene C2h4 Home Work Help Learn Cbse Forum
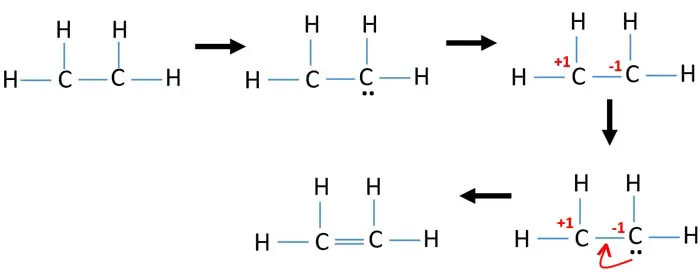
Ethene C2h4 Lewis Structure Hybridization
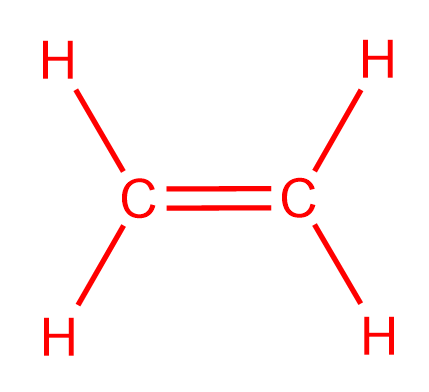
Draw The Lewis Structure For The C2h4 Ske Clutch Prep
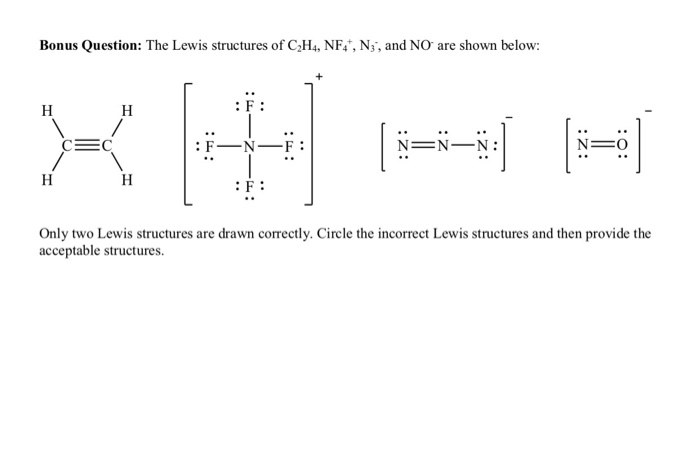
Bonus Question The Lewis Structures Of C2h4 Nf N Chegg Com
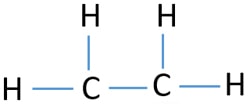
Ethene C2h4 Lewis Structure Hybridization
Lewis Electron Dot Structures Ck 12 Foundation

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Lewis Structure Of C2h4 Biochemhelp

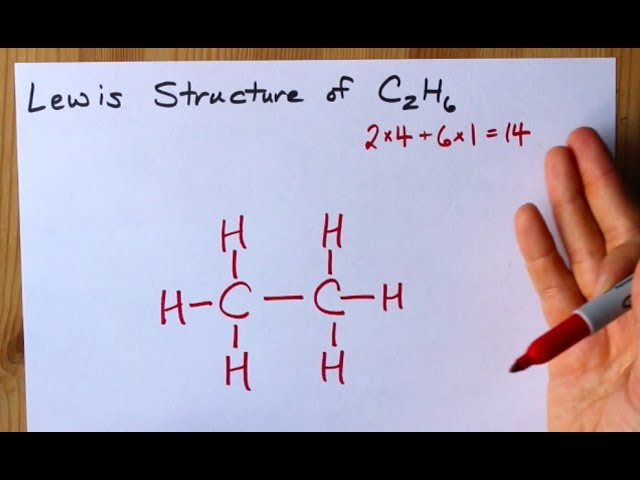
Lewis Structure Of C2h6 Ethane Youtube
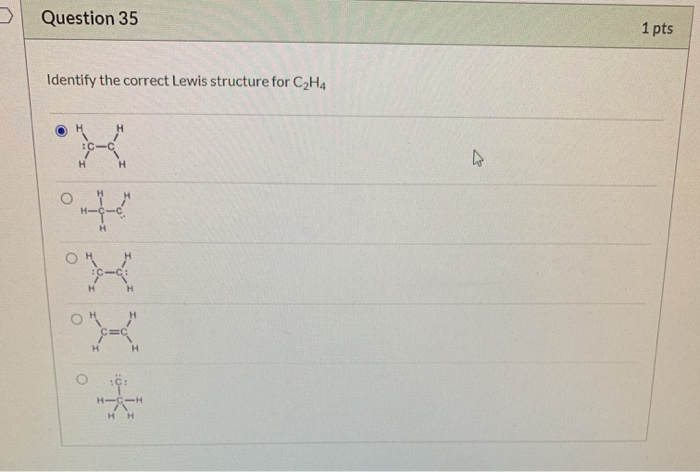
Question 35 1 Pts Identify The Correct Lewis Chegg Com
