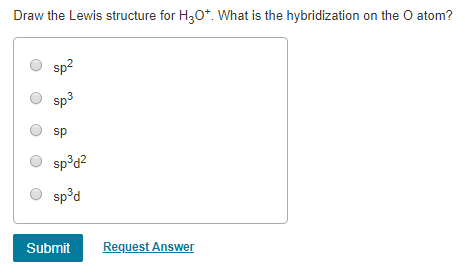
Draw The Lewis Structure For H3o+. What Is The Hybridization Of The O Atom
To understand why we can first draw out the Lewis structure of H3O H 3 O hydronium. 1 Group s1 Hybridization.
What Is The Hybridization Of H In H3o Quora
107 58 Draw the Lewis structure for SO3.

Draw the lewis structure for h3o+. what is the hybridization of the o atom. What is the hybridization on the O atom. Well finish by putting brackets around it like this here to show that its an ion and put a plus charge up here so everyone knows that its a positive ion a cation. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital.
What is the hybridization on the O atom. Three hydrogen atoms are bonded to oxygen so the choice of the monovalent atoms M 3As it is a cationic molecule thus C 1. A sp2 B sp3d2 C sp3d D sp3 E sp 43 Consider the molecule below.
Drawing the Lewis Structure for H 3 O For the H3O Lewis structure we first count the valence electrons for the H3O molecule using the periodic table. The hybridization of H3O hydronium ion is Sp3. When we are done adding valence electrons we check each atom to see if it has.
Give the hybridization for the O in H3O. Larger compounds have molecular units in which at least one atom is covalently bonded to multiple other atoms. See the answer See the answer See the answer done loading.
2D Draw the Lewis structure for Sho. Draw the Lewis structure for H3O. D spd E spd2 22 Draw the.
In hydronium ion the central atom is oxygen and it has 6 valence electronsThus by the method V 6. Once we know how many valence electrons there are in H3O we can distribute them around the central atom and attempt to fill the outer shells of each atom. What is the hybridization on the S atom.
NOCl CF 2 Cl 2 HCN. Put the least electronegative atom in the center. For the H3O Lewis structure we first count the valence electrons for.
Draw the Lewis structure for SO3. What is the hybridization on the O atom. XeO 2 F 2.
The hybridization of any molecule can be found using a formula. We have four groups on our central atom O. This problem has been solved.
What is the hybridization on the O atom. H2O H3O HCN. Determine the hybridization at each of.
A sp B sp2 C sp3 What is the hybridization on the S atom. H 2 S NCl 3 OH -. Steps for Writing Lewis Structures.
Simple way to understand the number of hybrid orbitals that we need is to find out how much groups are attached to the central atom Once we know the number of attached groups Here is simple rule to apply. Draw the Lewis structure for H 3 O. H ½ VM-CA Here H Hybridization V No.
What is the hybridization on the Br atom. A sp3d2 B sp3d C sp3 D sp2 E sp 42 Draw the Lewis structure for BrF5. Draw and explain the Lewis structure for H3O.
What is the hybridization on the S atom. The 3-dimensional geometrical structure of ammonium NH4 is referred to as Tetrahedral. 1 The oxygen O atom in H3O H 3 O is sp3 s p 3 hybridized.
Chemistry is all about exceptions after all. Thats the Lewis structure for H3O the hydronium ion. A sp B sp3 C sp2 D sp3d E sp3d2 23 Use Lewis theory to determine the chemical formula for the compound formed between Al and O A Al302 B Al203 C AlO2 D Question.
What is the hybridization on the O atom. List the number of sigma bonds and pi bonds in a. B and thanks for watching.
What is the. Find the total valence electrons for the molecule. A step-by-step explanation of how to draw the H2CO Lewis Structure Formaldehyde.
What is the hybridization on the O atom. What is the hybridization of O. A step-by-step explanation of how to draw the H3O Lewis Structure Hydronium Ion.
What is the hybridization on the O atom. Nitrogen having 5 valence shell electrons along with 4. Thus we will be able to say hybridization of H3O is Sp3.
H always goes outside. Determine the electron geometry eg molecular geometry mg and polarity of SO3. A molecule containing a central atom with sp3d hybridization has an _____ electron geometry.
107 57 Draw the Lewis structure for H3O. Lewis structure rules are almost the same for all molecules although some exceptions exist here as well. The central atom here is oxygen which is hybridized.
Draw the Lewis structure for H3O. So H ½ 6 3 1 4. Draw the best Lewis structure for BrO4 and determine the formal charge on bromine.
Formaldehyde H2CO is the simpiliest of a class of functional groups call. 1 Lone pair of electron. One can draw the 3-dimensional structure of an atom once they have the Lewis Structure of an atom.

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
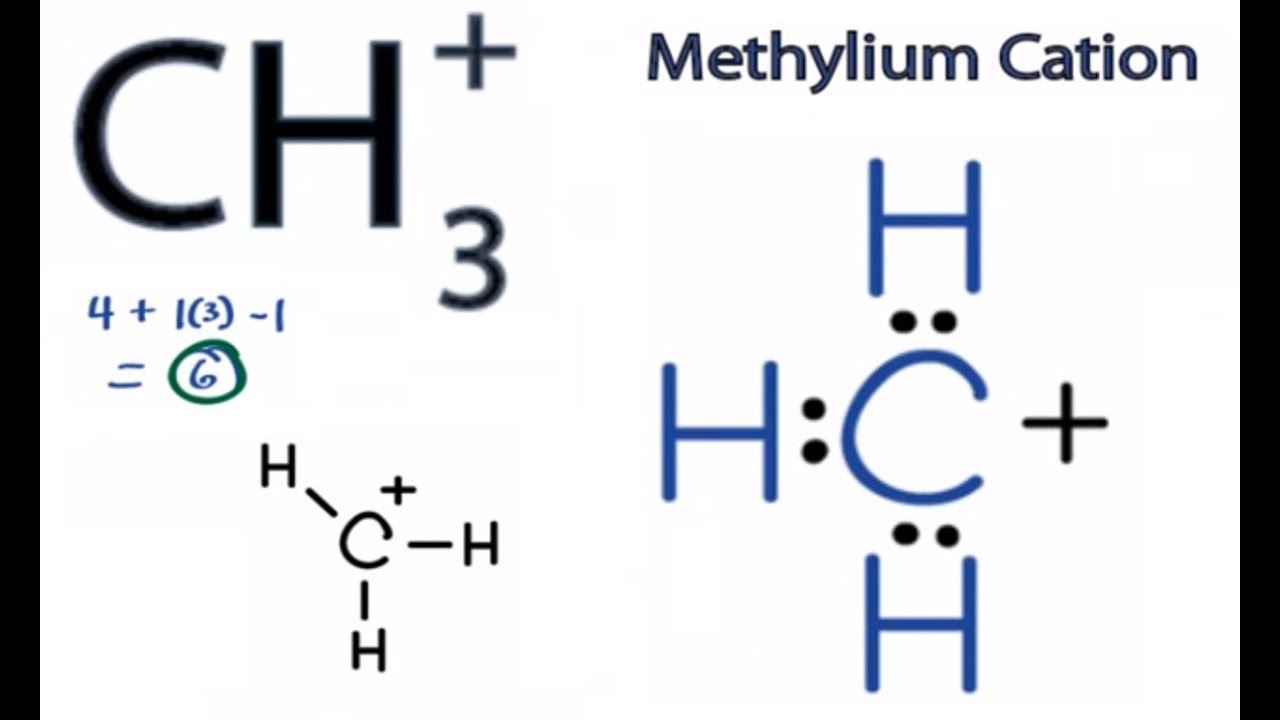
Ch3 Lewis Structure How To Draw The Lewis Structure For Ch3 Youtube
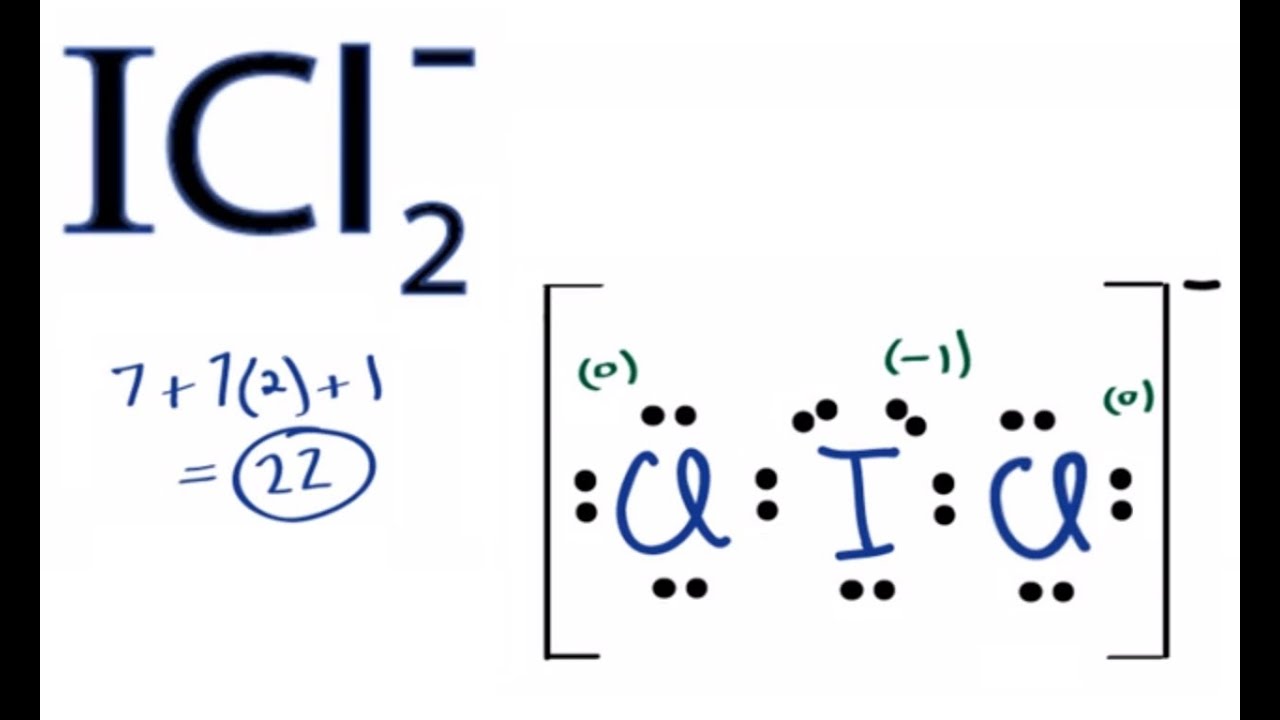
Icl2 Lewis Structure How To Draw The Lewis Structure For Icl2 Youtube
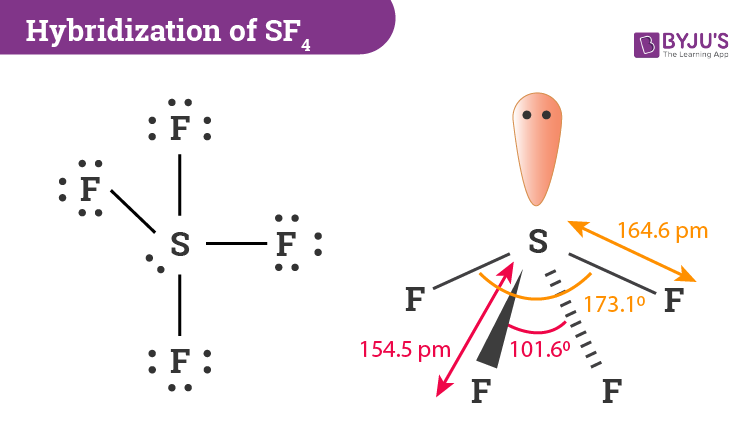
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

Hybridization For Nh4 Description Of Hybrid Orbitals For Nitrogen Youtube
Cs2 Lewis Structure Hybridization Polarity And Molecular Shape

Predicting Bond Angles Youtube

Hybridization Of I3 Hybridization Of Iodine In I3 Ion

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

Lewis Structure Hybridization Co3 2 Youtube
Xef2 Lewis Structure Polarity Hybridization And Shape

H3o Molecular Geometry Shape And Bond Angles Youtube

What Is The Shape Of H3o Ion Quora
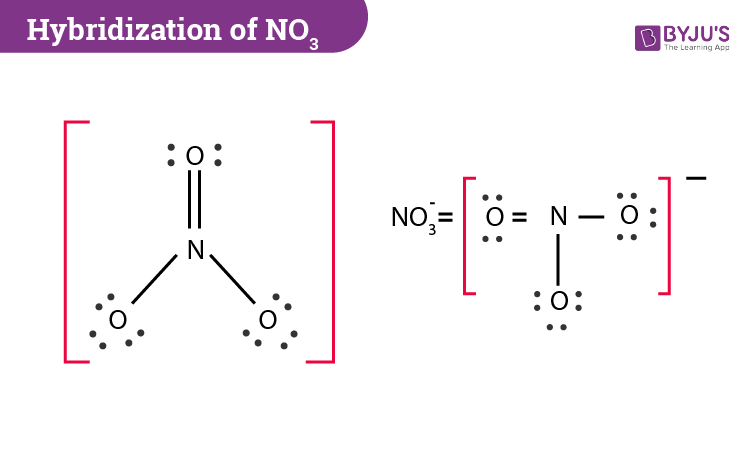
Hybridization Of No3 Hybridization Of N And O In Nitrate
Draw The Lewis Structure For H3o What Is The Chegg Com

What Is The Hybridization Of No3 Quora
What Is The Hybridization Of H In H3o Quora
