How To Find Hybridization Of C2h2
21 The orbital hybridization on the carbon atoms in C2H2 is. If the steric number is 2 sp.
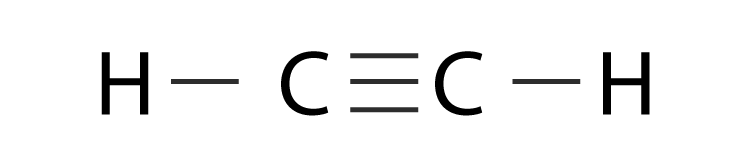
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
One of the hybrid orbitals forms a sigma-bond to hydrogen and the other form a sigma-bond between the.

How to find hybridization of c2h2. Z 3 sp2. So now lets go back to our molecule and determine the hybridization states for all the atoms. There are two methods to find out hybridization of atoms.
See the answer Explain the hybridization of carbon in C2H2 using electron configuration diagrams of the original atoms excited staes and hybridized staes. The number of lone pairs on nitrogen atom v - b - c 2 5 - 4 - 1 2 0. The 2s orbital in each carbon hybridizes with one of the 2p orbitals and forms two sp hybrid orbitals.
Find an answer to your question What type of hybridization is needed to explain why ethyne C2H2 is linear. Assign hybridization and shape of molecule. So for the 2 σ σ bonds it forms hybridized orbitals by mixing s and p orbitals.
Observe how many sigma bonds and lone pairs exist around a carbon atom and add them. Atomic orbitals of almost equal energy combine to form new molecular orbitals. Steric number no.
Now the two p. If the steric number is 3 sp2. Carbon has one s and three p orbiltals in its outer most shell.
C 2 H 2 has a linear shape given its molecular geometry is linear and all the atoms are arranged symmetrically. If structure is provided hybridization can be calculated on the basis of number of sigma bonds and lone pairs. How to find hybridization.
One sp hybrid orbital of one carbon atom overlaps axially with sp hybrid orbital of the other carbon atoms to form C-C sigma bonds. Z 2 sp. In Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions.
If these are half-filled they may form bonds with other atoms having half-filled atomic orbitals. Z6 sp 3 d 2. Of lone pairs 4 0 4.
In the formation of C2H2 the carbon atom needs extra electrons to form 4 bonds with hydrogen and other carbon atoms. C l O X 2 has 2 σ bonds 1 lone pair 2π bonds and 1 odd electron. Sp hybridization is also called diagonal hybridization.
C2 SN 3 three atoms connected therefore it is sp2. C2H2 Hybridization In the formation of ethyne molecule both the carbon atoms undergo sp-hybridization having two unhybridized orbital ie 2py and 2px. 21 The orbital hybridization on the carbon atoms in C2H2 is.
The new molecular orbital formed is known as hybrid orbital and this phenomenon is known as hybridization. Thus sp- hybridization arises when one s and one p orbital combine to form two sp-orbital with 180 bond angle and linear shape to the molecule. This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as a sp hybridized orbital.
Z 5 sp 3 d. Hybridisation is equal to number of σ bonds lone pairs. Calculate the number of lone pairs.
C1 SN 3 three atoms connected therefore it is sp2. Can I decide the hybridization of carbon atoms by looking the lewis structure of acetylene. Of σ-bonds no.
C 2 H 2 acetylene or ethyne. The percentage of s and p are 50. Calculate the steric number of central atom.
Important Points To Remember. The bond angle in C 2 H 2 is 180 degrees. Therefore hybridization of carbon atom is sp 2.
Of sigma bonds lone pair of central atom. Each sp hybridized orbital has an equal amount of s and p character ie 50 s. There are only two sigma bonds around a carbon atom.
Z 4 sp 3. Find the hybridization as well identify the pπ-pπ as well as pπ-dπ bonds in C l O X 2. The carbon atom is sp hybridized.
What is the structure of C2H2 on the basis of hybridization. All the atoms are arranged symmetrically as both the Carbon atoms form a single bond with Hydrogen atoms. None of these.
Since we consider odd electron a lone pair like in N O X 2 therefore hybridisation is coming to be s p X 3. As a result one 2s 2 pair is moved to the empty 2pz orbital. Hence the Carbon atom has sp hybridization in the C 2 H 2 molecule.
What Is The Structure Of C2h2 On The Basis Of Hybridization Quora

What Is The Difference Between 1s And 2s Orbital Updated Different Push Pin Update
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Valence Bond Theory Chemistry For Non Majors Theories Bond Chemistry

Pin Pa 100 Steps To Sat Ii Chemistry

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation
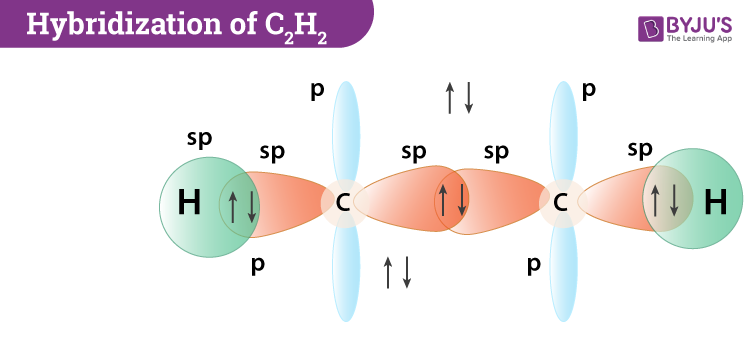
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

Ethylene Sp2 Hybridization Mario Characters Illustration Chemistry

Sp3 Sp2 And Sp Hybridization In Organic Chemistry With Practice Problems Chemistry Steps

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Welcome To Learnapchemistry Com Ap Chem Ap Chemistry Molecular Geometry
How To Detect The Hybridization Of Acetylene Quora
Answer In Organic Chemistry For Tj 92360

How To Determine The Hybridization Of An Atom Sp Sp2 Sp3 Sp3d Sp3d2 Practice Problem Example Youtube

Chemistry Molecular Structure 38 Of 45 Hybridization And The Triple Bond 2 Pi Bonds Youtube

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula

