How To Find Lone Pairs In A Lewis Structure
And other are non-bonding electrons or lone. Final Lewis structure for carbon dioxide.
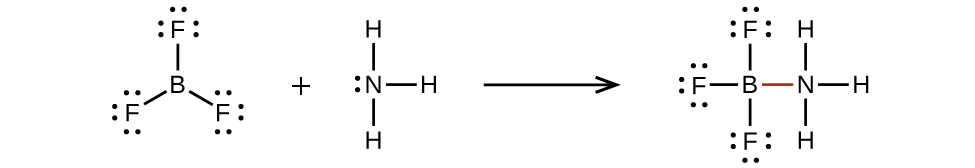
7 3 Lewis Symbols And Structures Chemistry
The total number of valence pairs can be found by adding all the pi and sigma bonds the compound makes with lone electrons.
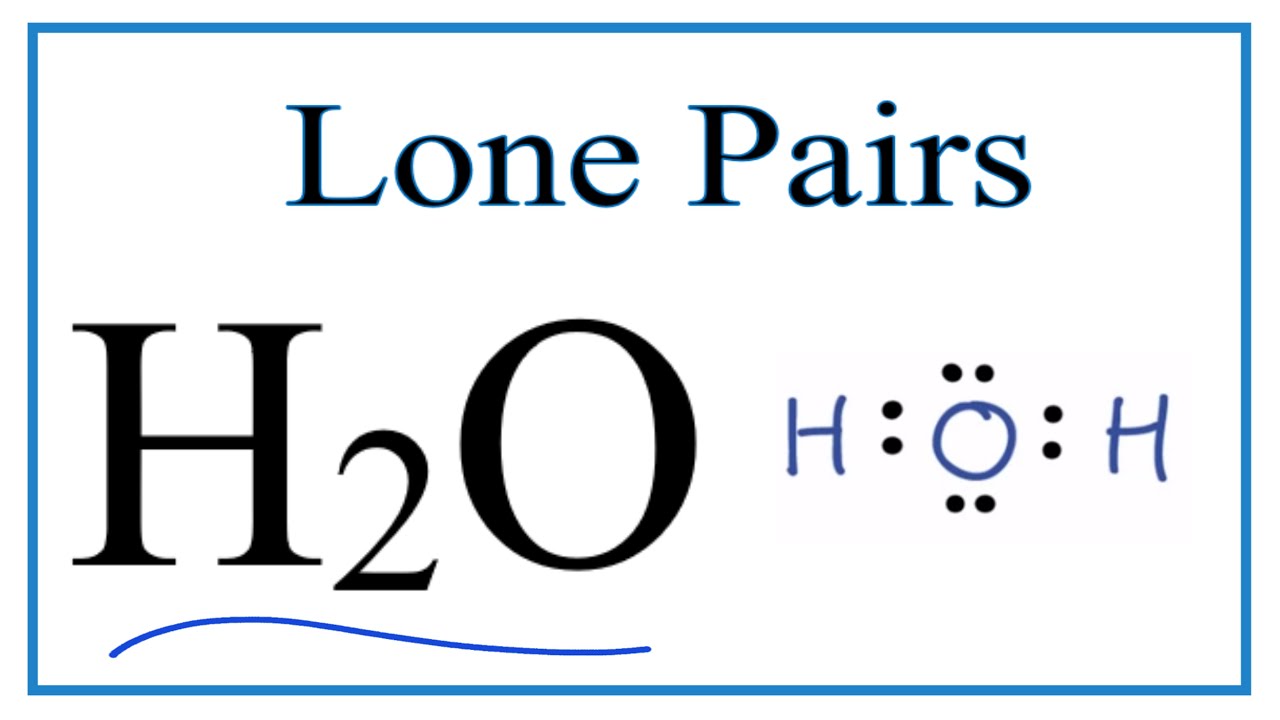
How to find lone pairs in a lewis structure. From three oxygen atoms one oxygen atom has one lone pair. 4 Lone Pairs e. Here the total pairs are 13 in number.
Also one oxygen atom has a -1 charge and another oxygen atom has a 1 charge. A How many bonding pairs of electrons are in the Lewis Structure. Divide the number of VEs not in bonds from Step 3 by 2 to find the number of LPs.
Find the number of lone pairs on the central atom by subtracting the number of valence electrons on bonded atoms Step 2 from the total number of valence electrons Step 1. As we see in the ClF3 lewis structure Chlorine which is the central atom contains 2 lone pairs. O2 O 3 ОО O 1 04.
According to the lewis structure of Ethene there is no lone pair present on the central atom. See the answer See the answer See the answer done loading. Draw single bonds between.
Show transcribed image text. Select c What is the molecular geometry of OF2. In the lewis structure of NH 3 you can see there are one double bond and one single bond between oxygen atoms.
Determine the central atom which is the least electronegative atom. 10 Lone Pairs c. We could use a lone pair on either O or Cl.
8 Lone Pairs b. In the lewis structure of Nitrogen trifluoride NF 3 there are three N-F bonds and one lone pair on nitrogen atom. There are two types of electrons constituting the bond formation in any compound.
6 Lone Pairs f. Lone electron pairs of an atom in the Lewis structure of a molecule are the electron pairs that are placed on those sides of the symbol of the atom on. Find the Number of lone pairs present on the central atom of the ClF3 lewis structure.
Or we can find lone pair in ClF3 by using the direct formula. Find out the Central atom. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in.
Identify number of lone pairs in Lewis Dot Structure. To find the valence pairs we will divide the total number of valence electrons by 2 because a pair can be made with the help of 2 electrons. How many lone pairs of electrons are on the central atom in the Lewis structure for PCI3.
For example If the number is 0 there are no lone pairs on the central atom. Select b How many lone pairs of electrons are present on the central atom in the Lewis structure. Find the Number of lone pairs present on the central atom of the C2H4 Lewis structure.
Covalent bonds are indicated as dashes and lone pairs of electrons are shown as pairs of dots. The Lewis structure is nothing but a representation of how the electrons participate in the bond formation to form the particular compound. 2 Lone Pairs d.
The covalent bonds between the oxygen and carbon atoms each use two electrons from the oxygen atom and two from the carbon. How many lone pairs of electrons would be shown in the Lewis structure of CO. First of all we need to find how many lone pairs ClF3 central atom contains.
Another one has two lone pairs and remaining one has three lone pairs. These are bonding pair of electrons which take part in forming the bond. How many lone pairs of electrons are on the central atom in the Lewis structure for PCI3.
To find lone pairs in a molecule figure out the number of valence electrons of the atom and subtract the number of electrons that have participated in the bonding. Each fluorine atom has three lone pairs. To confirm it you can also use the formula to find lone pairs.
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons leaving 2 electrons to place as a lone pair on nitrogen. This problem has been solved. LP VE.
Because this Lewis structure has only 6 electrons around the central nitrogen a lone pair of electrons on a terminal atom must be used to form a bonding pair. Each fluorine atom has three lone pairs. Draw the correct Lewis structure of OF2 and then use it to answer the questions below.
However remember that the lone pairs are pairs and therefore if yo. O2 O 3 ОО O 1 04. The lone pair is also called the unshared pair.
Count for the valence electron in the compound by adding all the elements valence electrons. In carbon dioxide each oxygen atom has two lone pairs of electrons remaining.

Lewis Structures In Covalent Bonds Valence Electrons Are Distributed As Shared Or Bond Pairs And Unshared Or Lone Pairs H Cl Shared Or Bond Pair Ppt Video Online Download
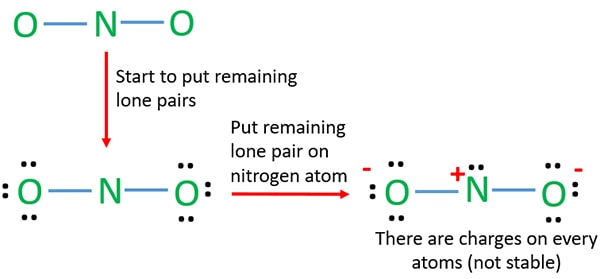
Lewis Structure Of No2 Nitrite Ion
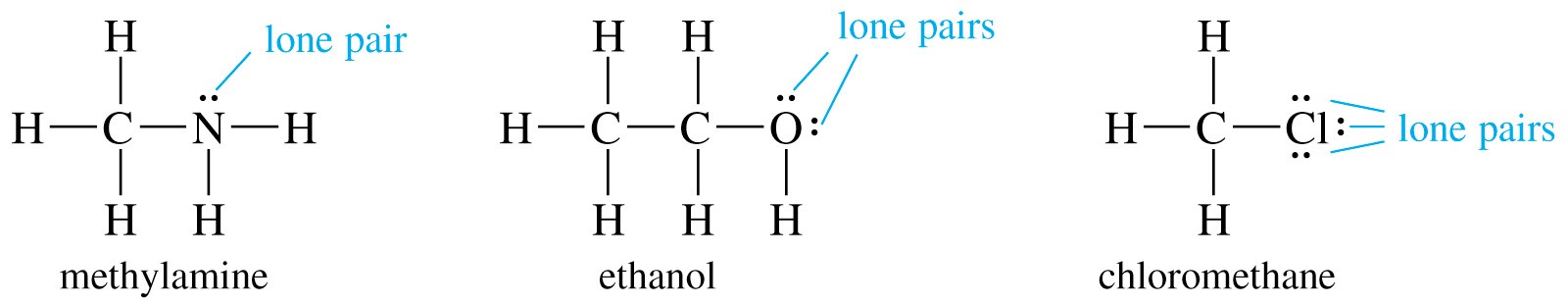
1 6 Lewis Structures Chemistry Libretexts

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Number Of Lone Pairs And Bonding Pairs For Of2 Oxygen Difluoride Youtube

Lewis Structure Brilliant Math Science Wiki

How To Find Lone Pairs Of Electrons Of Any Atom Trick To Find Lone Pair Of Electrons Youtube
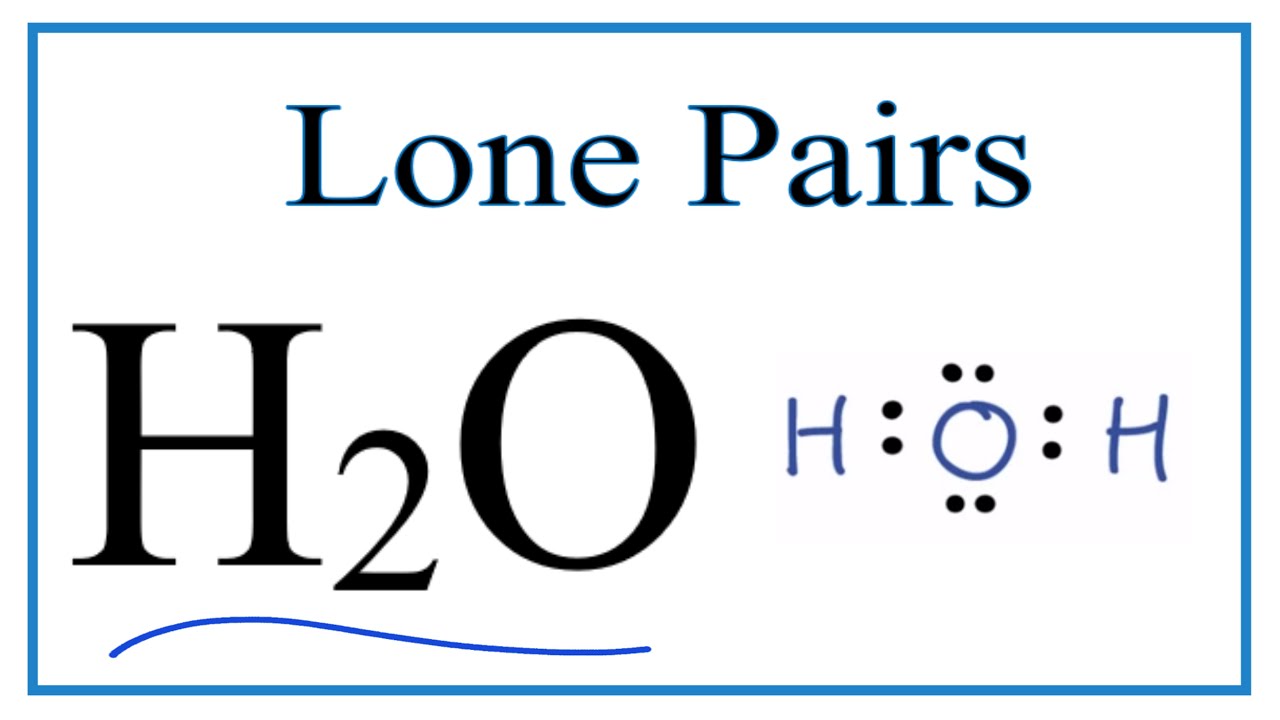
Number Of Lone Pairs And Bonding Pairs For H2o Water Youtube

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube

How To Find The Lone Pair Quora
What Is The Easiest Way To Find Bond Pair And Lone Pair In Chemical Bonding Quora

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube

Number Of Lone Pairs And Bonding Pairs For Co2 Carbon Dioxide Youtube

Number Of Lone Pairs And Bonding Pairs For Nh3 Ammonia Youtube

How To Determine The Number Of Lone Pairs Chemistry Steps

Identify Number Of Lone Pairs In Lewis Dot Structure Youtube

1 Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Co 2 2 Draw The Main Lewis Structure Of Nof 3 Determine The Number Of Bonding