Lewis Electron Dot Structure For C2h4
Youre Going to Have to Do This More Than Once. Here carbon has a 4 valence electron.
Lewis Structure Of C2h4 Biochemhelp
To complete a Lewis electron-dot diagram including any lone non-bonding pairs of electrons when given an array of atoms arranged to represent ethanol.

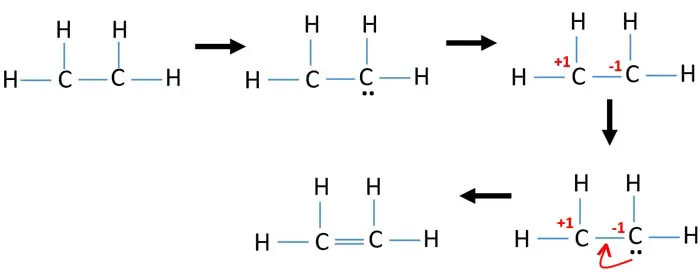
Lewis electron dot structure for c2h4. Xenon has 4 bonding electron pairs and 2 nonbonding pairs so the molecular geometry is square planar. Figure 3 illustrates one pitfall in trying to draw a valid Lewis electron dot structure for ceC2H4. Alternatively a dot method can be used to draw the lewis structure.
C2H4 Lewis Structure Ethene. C H O N S P F Br Х More Part C HCO3 Draw the Lewis dot structure for H2CO3. Part aii assessed knowledge of structural isomers by asking students to draw a complete Lewis electron-dot diagram for the isomer of the compound drawn in part ai.
Is it polar or nonpolar. What is the electron dot structure of cyclohexane. What is the shape of C2H4.
In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. As per the Ethene Lewis dot structure Four CH sigma bonds are present and one CC double bond1 sigma 1 pie bond. Include all lone pairs of electrons.
Note that the C2H4 Lewis dot structure involves. Use information from step 4 and 5 to draw the lewis structure. To know the reason why c2h4 has two ch and four h molecules.
How many shared pairs of electrons are in the lewis dot structure of C2H4. Electron dot structure of C2H4 H2CCH2. The key to understanding how to distribute the valence electrons is to.
To do that we always count our valence electrons up first. What is the Lewis structure of benzene. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
The electron-dot structure of cyclohexane is shown below. What is the electron dot formula of C2H4. The usual structural representation for benzene is a six carbon ring represented by a hexagon which includes three double bonds.
Oxygen contains 6 valence electrons which form 2 lone pairs. What are the two main components of hexanes. Transcribed Image Textfrom this Question.
Lewis Structure of CO2. Electron Dot Structure for ethane C2H4. Electron dot structure of C 2.
H O N S P F Br Part D C2N2 Draw the Lewis dot structure for C2N2. Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. Lets take a look.
Its C2H4 and we want to write the dot structures for ethene. Lewis dot structure of C 2 H 4. Drawing the Lewis dot structure for C2H4 ethene and answer the questions below.
Draw the electron-dot structure for C2H4. What is its molecular shape. Drawing electron dot structures always involves trial and error even when you follow the rules.
The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. When you look at the lewis dot structure of this molecule there are no lone pairs of electrons in its structure. The electron dot structure is drawn using Lewis-dot structure.
Hence total shared pairs of electrons in the Lewis dot structure of C2H4 is 12. According to electron-dot structure there are 36 number of bonding electrons and 0 number of non-bonding electrons. Include all lone pairs of electrons.
It means four CH bond has 8 shared pairs of electrons and CC bond has 4 shared pairs of electrons. The Lewis electron dot structures of a few molecules are illustrated in this subsection. If we come way over here to Hydrogen its in group 1.
This means that the carbon atoms share 4 electrons. C2H Draw the Lewis dot structure for C2H4. This means that the carbon atoms share 4 electrons.
Calculate the total valence electrons in the molecule. Then learn how to predict the shape of a molecule by applying the VSEPR theory to the Lewis dot. There are two triangles overlapping each other as we can see in the diagram.
Since it is bonded to only one carbon atom it. Watch the video of Dr. What is the chemical formula for methane.
Electron dot structure of C2H4 H2CCH2. C C H 2. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. It has 1 valence electron. Polar protic vs polar aprotic vs nonpolar.
Carbon has 4 valence electrons hydrogen has 1 valence electron. The central atom of this molecule is carbon. It consists of two carbon molecules and 4 hydrogen molecules.
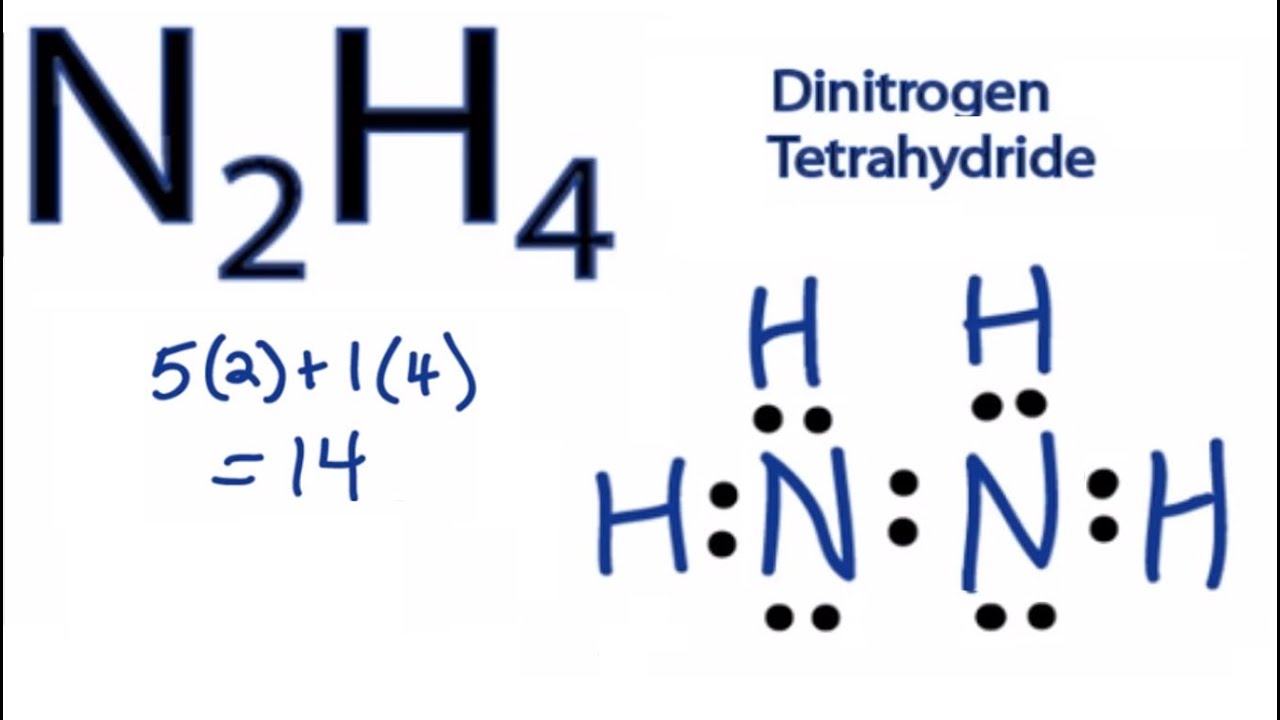
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
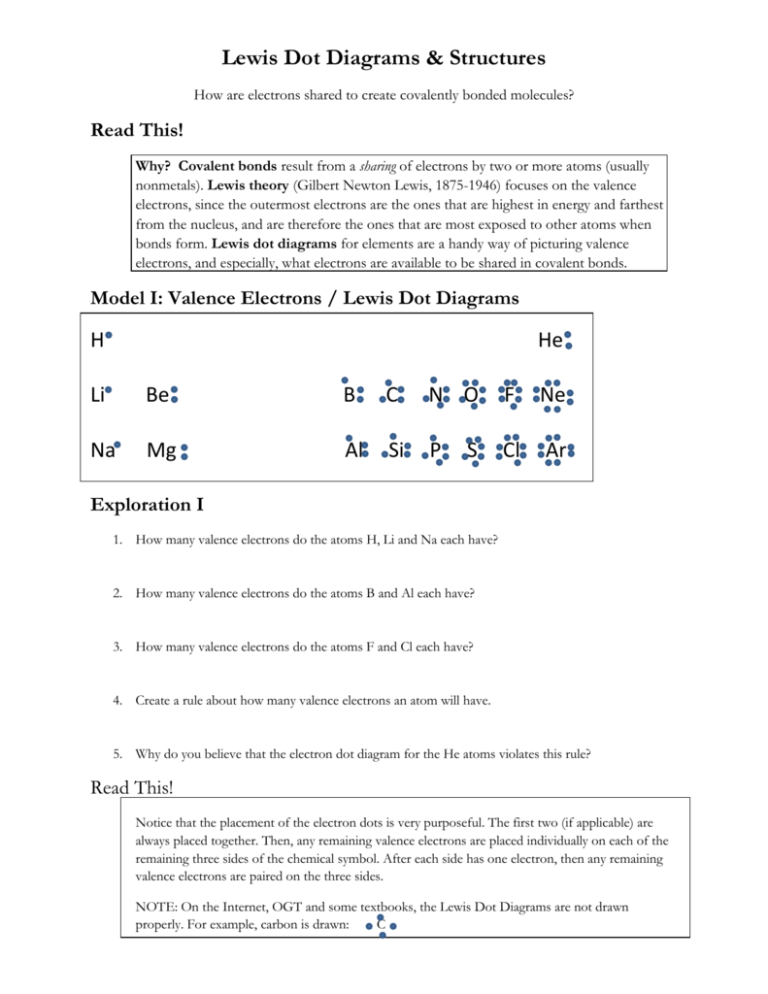
Lewis Dot Diagrams Structures H He Li Be B C N O F

Part A Draw A Lewis Dot Structure For Pho Clutch Prep
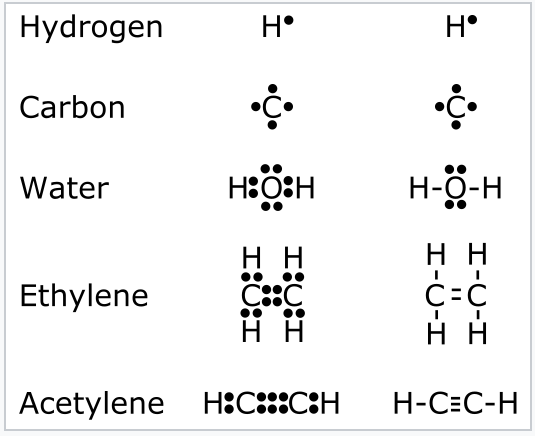
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts
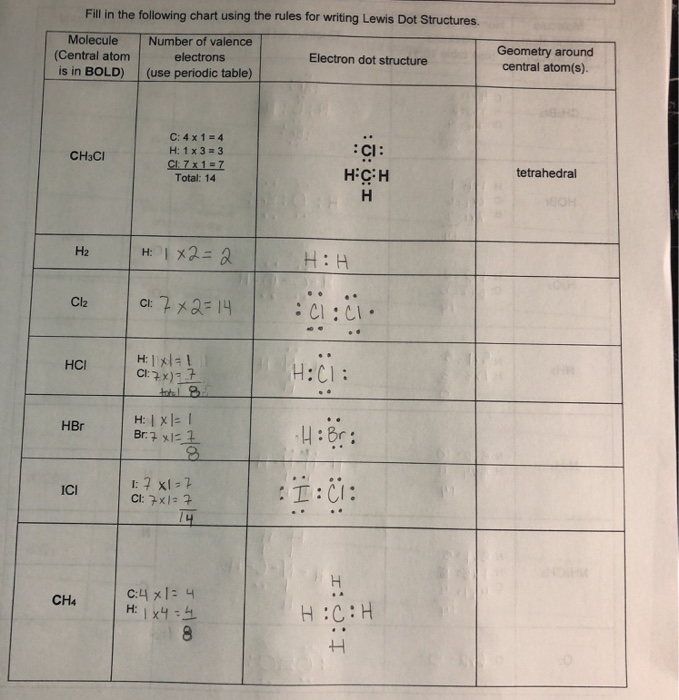
Fill In The Following Chart Using The Rules For Chegg Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Lewis Electron Dot Structures Ck 12 Foundation

7 3 Lewis Symbols And Structures Chemistry

Write The Electron Dot Structure Of Ethene Molecule C2h4
Lewis Electron Dot Structures Ck 12 Foundation

Based On Your Answers To Parts A B And C Clutch Prep

Draw An Electron Dot Structure Of The Following Molecules Without Showing The Circles A Methane B Brainly In
Lewis Electron Dot Structures Ck 12 Foundation

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Electron Dot Structure Of Ethane Quora
Ethene C2h4 Lewis Structure Hybridization

Draw The Electron Dot Structure Of Ch4 Brainly In

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In



