Lewis Structure For Pcl3br2
Identify the true statement about Lewis structures. Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure.

Bell Work P 263 Co2 H2s Draw These Lewis Structures And Name The Shape Ppt Download
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure.

Lewis structure for pcl3br2. Draw the Lewis structure for the molecule. Geometry Bond Angles and Polarity Chlorine pentafluoride ClF5 is a good oxidizer and flourinator. Select all the correct answers.
Carbon EN 25 is less electronegative than Bromine EN 28 and hydrogen can only make 1 bond so carbon is the central atom. In PCl3F2 central Phosphorus atom is sp3d hybridized. PCl3Br2 is a nonpolar molecule.
XeO 2 F 2. H 2 S NCl 3 OH -. Vinyl chloride IUPAC name chloroethene is a man-made industrial chemical used mostly for manufacturing of polyvinyl chloride PVCPiping floor covering furniture automobile parts and packaging made of PVC are widely used throughout the world.
Cl-P-Cl at 120. A single bond in a Lewis structure represents 2 electrons. Drawing the Lewis Structure for C 2 H 2 Br 2.
Draw the Lewis structure for the molecule. Put two electrons between atoms to form a chemical bond. 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3.
Select all the correct answers. Lewis electron structures give no information about molecular geometry the arrangement of bonded atoms in a molecule or polyatomic ion which is crucial to understanding the chemistry of a molecule. Sometimes CIF5 is used as a rocket oxidizer.
Unread Mar 2 2002 10426 AM 3202. Note that Hydrogen only needs two valence electrons to. Identify the true statement about Lewis structures.
Put the least electronegative atom in the center. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Br 2 Lewis structure. H always goes outside.
See the related link. With C 2 H 2 Br 2 there are only single bonds. What is the most likely structure of PCl3Br2 and explain why its the most likely structure.
What is the structure for PCl3Br2 and 3 isomers. Obtain the molecular geometry from the directions of the bonding pairs for this. Determine the number of electron groups around the indicated atom.
Determine the arrangement of these electron pairs about the central atom. Calculate the total number of valence electrons present. If resonance exists give all the resonance structures as well.
Were being asked to identify the bond angle in ICl 4 Since we dont know the Lewis structure for ICl 4 we need to do the following steps. The Lewis structure is useful for predicting the molecules geometry and showcases. The axial position of metal has less s-character and the equatorial position of metal has more s-character.
Five sp3d- hybridized orbitals are arranged in trigonal. Lewis explained the formation of chemical bonds based on the distribution of electrons. A step-by-step explanation of how to draw the PF3Cl2 Lewis Dot StructureNote that Phosphorous P can hold more than 8 valence electrons it can have an exp.
The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. A single bond in a Lewis structure represents 2 electrons. Steps for Writing Lewis Structures.
Write the electron-dot structure from the molecular formula. Find the total valence electrons for the molecule. Determine the central atom in this molecule.
Determine the central atom in this molecule. Give a Lewis structure for eqClBr_2 eq eqCl eq is the central atom. In case of sp3 hybridization.
What is the structure for PCl3Br2 and 3 isomers. Clearly one would like to know quite a bit about the chemical nature of this compound to predict how it. Calculate the total number of valence electrons present.
Emma Popek in Sampling and Analysis of Environmental Chemical Pollutants Second Edition 2018. It is a volatile liquid that reacts with water and releases HCl gas. The ligand which are s-phile s-lover will tend to occupy equatorial position and the ligands which are s-nonphile will tend to occupy axial position.
A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure Phosphorus TrichlorideFor the PCl3 structure use the periodic table to find the tot. NOCl CF 2 Cl 2 HCN. Determine the number of electron pairs both bond and lone pairs around the central atom.
The Br-P-Br would be 180 and the other would be 90. For Nonpolar C2H2Br2 one isomer. PCl3 Molecular Electron Geometry Lewis Structure Bond Angles and Hybridization.
Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. It states that a more electronegative atom prefers to bond with that hybridized orbital which has less s-character. A double bond in a Lewis.

How To Determine The Structure Of Pcl3br2 Quora
What Is The Structure Of C6h10 Quora
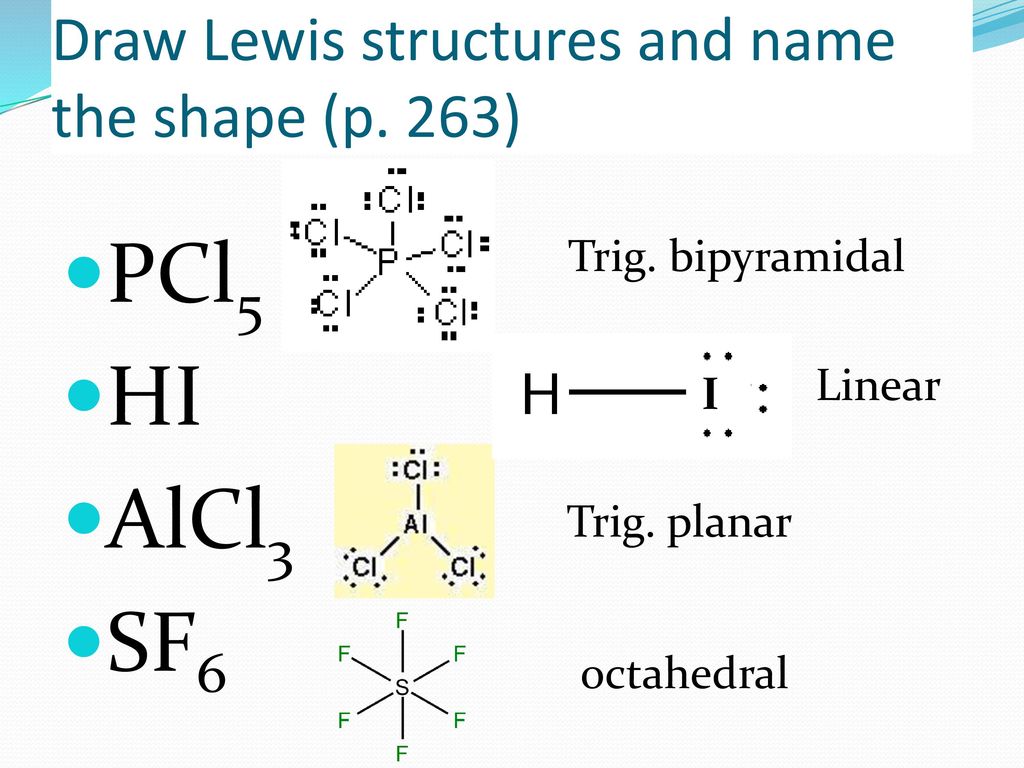
Bell Work P 263 Co2 H2s Draw These Lewis Structures And Name The Shape Ppt Download

There Are 3 Different Possible Isomers Of Clutch Prep
What Is The Hybridised Structure Of Pcl3f2 Quora
What Is The Structure Of Ncl3 Quora
How To Determine The Structure Of Pcl3br2 Quora

Pcl3br2 Is A Nonpolar Molecule Based On This Information Determine Bond Angle Of Cl P Cl Br P Br And Cl P Br Study Com
What Is The Structure Of Bcl3 Quora
How To Determine The Structure Of Pcl3br2 Quora
What Is The Bond Angle Of Pcl3

The Correct Structure Of Pcl3br2 Is
How To Determine The Structure Of Pcl3br2 Quora
Why Is Pf3cl2 A Polar Molecule But Pcl3f2 Is Non Polar Quora
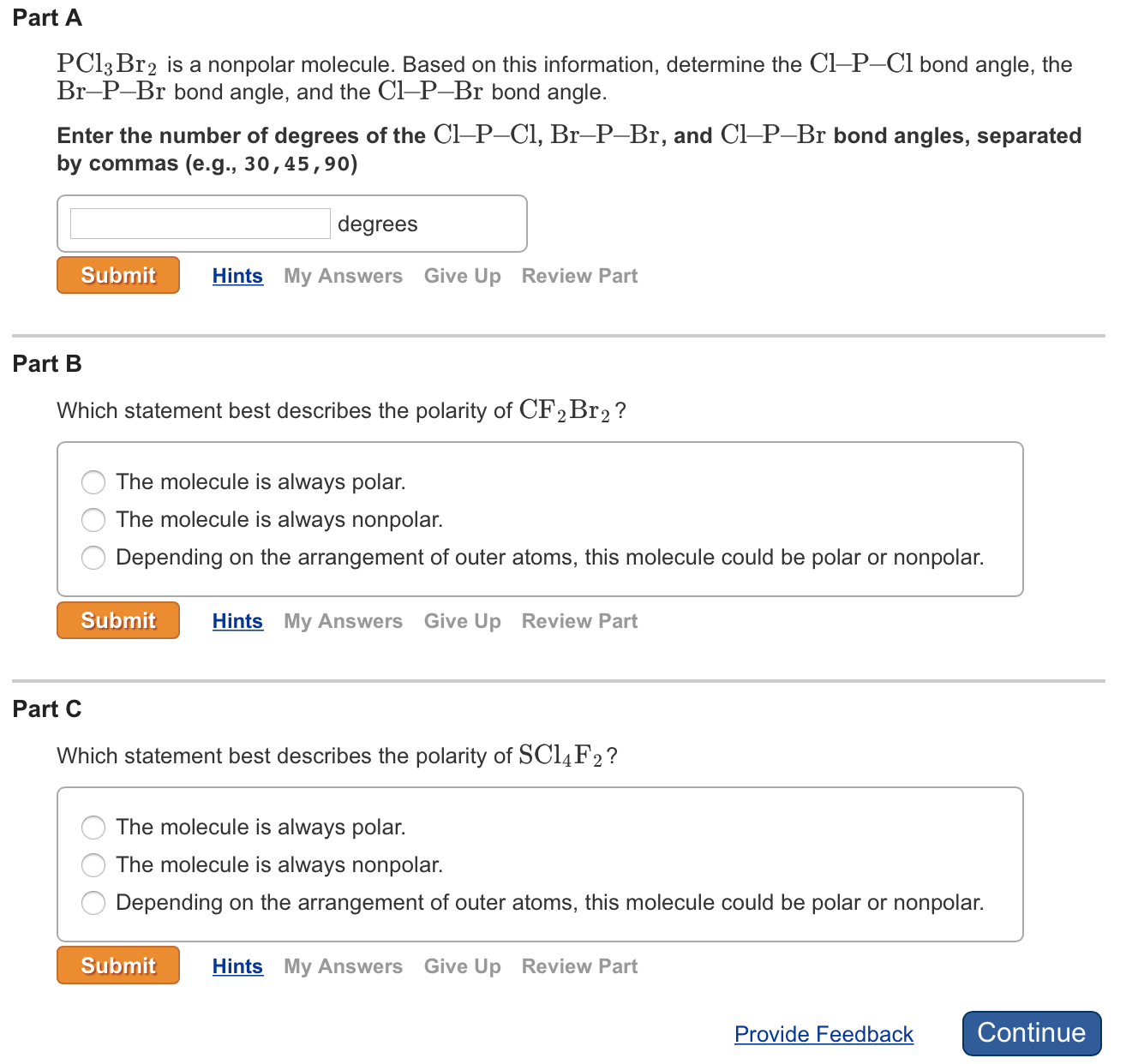
Part A Pcl3 Br2 Is A Nonpolar Molecule Based On This Chegg Com

How To Determine The Structure Of Pcl3br2 Quora
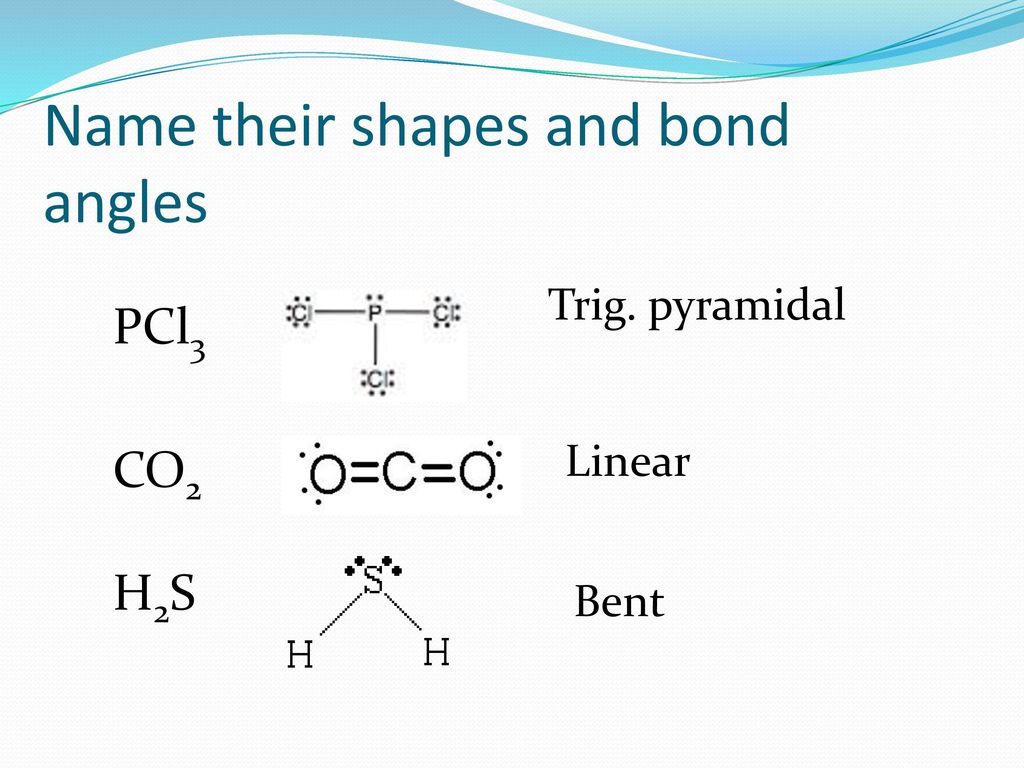
Bell Work P 263 Co2 H2s Draw These Lewis Structures And Name The Shape Ppt Download

What Is The Structure Of Sf6 Quora
What Is The Structure Of Bcl3 Quora