Pcl3 Electron Group Geometry
Electron group geometry depends on the number of electron groups around the central atom. Pcl3 Lewis Structure Electron Geometry What is the molecular geometry of PCL3.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Why is BCl3 electron deficient.

Pcl3 electron group geometry. Best Pcl3 Electron Domain And Molecular Geometry ImageConsider the following species when answering the following questions. Spread the love. Is PCl3 a resonance.
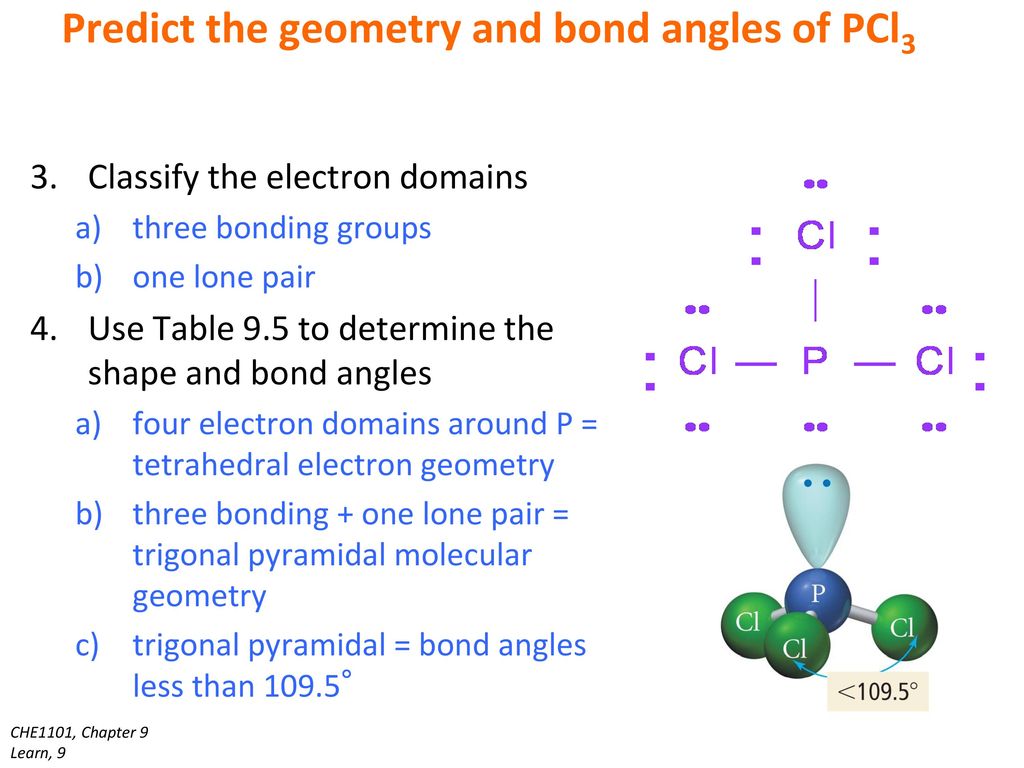
What are the bond angles around the central atom. Looking at the PCl 3 molecular geometry it is trigonal pyramidal with a bond angle of approx. What is the molecular geometry around the central atom.
BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm. Why is PCl3 tetrahedral. How many electron groups are around the central atom.
Chlorine belongs to group 7 elements with 7 as valence electrons. Note that Boron can have a full outershell with only six valence electrons. The central P atom has one lone pair of electrons and three bond pairs of electrons.
It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. From Figure PageIndex3 we see that with three bonding pairs around the central atom the molecular geometry of BCl 3 is trigonal planar as shown in Figure PageIndex2. Count the number of electron groups and identify them as bond pairs of electron groups or lone.
In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. The shape of a PCl3 molecule is Trigonal pyramidal. This is due to PCl3 being sp3 hybridized.
B and thanks for watching. The covalent and ionic bonds are shown along. What is the shape molecular geometry of PCl3 The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom.
PCl3 Phosphorus Trichloride. So PCl3 does not exhibit resonance. The molecular shape of the Phosphorous trichloride PCL3 is trigonal pyramidal and electronic geometry is tetrahedral based on VSEPR theory.
The is mainly due to the disproportionate influence or greater repulsion of the phosphorus lone pair which makes it deviate from the ideal angle of 109 o. The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. What is the electronic and molecular geometry of the following molecules.
Study PCL3 Molecular Electron Geometry Lewis Structure Bond PCL3 Molecular Electron Geometry Lewis Structure Bond PCl3 Molecular Geometry Shape and Bond Angles YouTube. Sailboats for Sale on Lake Superior. PCl3 What is the electron geometry around the central atom.
As boron has three valence electrons it forms 3. Leave a Comment Business. A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond anglesLooking at the PCl3 Lewis structure we can see that the.
A quick explanation of the molecular geometry of pcl3 including a description of the pcl3 bond angleslooking at the pcl3 lewis structure we can see that. All electron groups are bonding pairs BP so the structure is designated as AX 3. Chlorine Cl is in group 7A and thus has 7 valence electrons and forms 1 bond to.
Phosphorus P is in group 5A and thus has 5 valence electrons and forms 3 bonds to complete its valence shell. Furthermore How do you find the molecular geometry Steps Used to Find the Shape of the Molecule Draw the Lewis Structure. If one or more of the bonding pairs of electrons is replaced with a lone pair the electron geometry does not change but.
Need help with chemistry. Electron group geometries refer to the five geometries. Read More About Hybridization of Other Chemical Compounds Hybridization Of XeF4.
Thats why NH2- Lewis Structure Molecular Geometry Polarity Hybridization Best D Pharmacy 1st Year Notes PDF Download 2021 Updated How Many Valence Electrons Does Carbon C Have. Linear trigonal planar tetrahedral trigonal bipyramidal or octahedral. Toggle navigation Main Menu.
Yes BCl3 is an electron-deficient compound. In PCl3 there are no pi electrons there are no double bonds. There are two dimensions of Phosphorous trichloride PCL3 one dimension contains electrons and the other dimensions contains bonds.

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

How Many Lone Pair Electrons Are There In Pcl3 Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
What Is The Bond Angle Of Pcl3 Quora
Https Www Mctcteach Org Chemistry C1020 C1020 Handouts Molecular 20modeling 20v 8 18 Pdf

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

What Is The Bond Angle Of Pcl3
What Is The Molecular Shape Of Pcl3 Quora

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Pcl3 Lewis Structure And Molecular Geometry Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Can The Molecular Geometry Of Phosphorus Trichloride Be Described Quora

What Is The Molecular Geometry Of Pcl3 Study Com
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

What Is The Molecular Shape Of Pcl3 Quora

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3