Pcl3 Lewis Structure Octet Rule
Please watch the following video on how to draw Lewis structures. We make 3 P C l bonds ie.

Lewis Structures For Covalent Molecules Step By Step Youtube
Write Lewis structures that obey the octet rule duet rule for H for each o 0211.
Pcl3 lewis structure octet rule. Lewis Structure of Magnesium Fluoride MgF2 Magnesium fluoride MgF2 is not a molecularcovalent compound. Electron dot structures of POCl3. This rule is applied to the main-group elements of the second period.
Is octet rule valid for n2. The s orbital contains 2 and the p orbital contains 6 for a grand total of 8 electrons maximum. In Each Case The First Atom Listed Is The Central Atom A.
ClO_2- SCl_2 PCl_2- D. Of the outer atoms. Connect the outer atoms to it by single bonds.
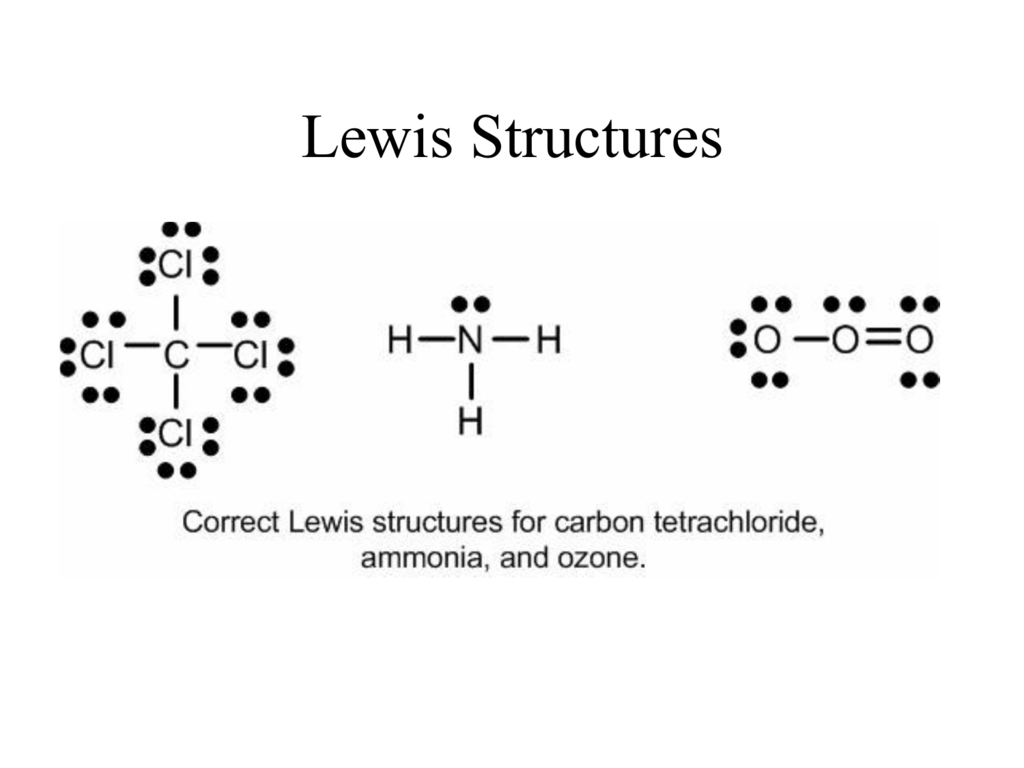
Connect each atom to the central atom with a single bond one electron pair. One exception to the octet rule is that in the real world some atoms in molecules or compounds do have fewer than eight octet valence electrons. Octet rule states that in forming compounds atoms gain lose or share electrons to give a stable electron configuration characterized by eight valence electrons.
Draw Lewis structures step by step. The octet rule is based on the number of valence electrons available for bonding and in the case of carbon nitrogen and oxygen the electrons come from the outermost s orbital and outermost p orbital. Therefore P 6n 2 V 6 5 2 32 0 So there is no double bond.
Considering Your Answers To Parts A B And C What Conclusions Can You Draw Concerning The. How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. Every atom should be in its least possible formal charge Also every atom in the lewis structure of PCl3 is fulling octet as they are having 8 electrons each after sharing.
Find the sum of valence electrons of all atoms 2. Step 3 4. And thus EACH chlorine atom has 3 non-bonding lone pairs and 2 electrons from the P C l bonds.
After fulfilling the electrons we can see the final lewis structure of PCl3. For which of the following can a valid Lewis structure be drawn without violating the octet rule. A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure Phosphoryl chlorideFor the POCl3 structure use the periodic table to find the tota.
Some atoms have fewer than eight valence electrons. Lastly to ensure the lewis structure is completely correct we need to check the octet of every atom and also their formal charge. There are three exceptions.
Second place the valence electron on the iodine and hydrogen atoms. Write Lewis Structures That Obey The Octet Rule For Each Of The Following Molecules And Ions. Writing Lewis Structures PCl 3 1.
The resonance Lewis electron dot structures of POCl3 are as follows. CO2 By signing up youll. Answer BF 3 is incorrect.
First the valence electrons are placed around the carbon atom. Hydrogen H in H 2. POCl_3 SO_42- XeO_4 PO_43- ClO_4- B.
To accommodate more than eight electrons sulfur must be using not only the ns and np valence orbitals but additional orbitals as well. 1 choose a center atom. Therefore it follows the octet rule.
Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. Distribute the remaining electrons as lone pairs on the terminal atoms except hydrogen completing an octet around each atom. NF_3 SO_32- PO_33- ClO_3- C.
Draw and explain the Lewis structure for eqPCl_3 eq. Is octet rule valid for pcl3. Fill the octet of.
What is the octet rule for Lewis structures. Exceptions to the octet rule include the following. A valid Lewis structure of cannot be drawn without violating the octet rule.
Keep track of the electrons. So a transfer of electrons occurs. 6 electrons in total one from the phosphorus and one from the chlorine atoms.
Some examples of these include. In case of SO2 as well the octet rule is being violated. Does this molecule have a central atom that violates the octet rule.
Write the Lewis structure for each molecule or ion. Thus we find that the octet rule is followed in case of only PCl3. In this post we discussed the method to construct the CH3I Lewis structure.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Nitrogen oxide No2 does not form octet rule. In PCl3 the octet for both phosphorus and Chlorine atoms is complete.
Magnesium has a low electronegativity it is a metal after all and fluorine has a high electronegativity it is a non-metal halogen and has the highest electronegativity of all atoms on the table. Where V 7 5 7 6 7 32 V is the number of valence electrons of the POCl3molecule. Subtract the number of bonding electrons from the total.
The is the least electronegative element that isnt hydrogen. The octet rule is based on the fact that each valence orbital typically one ns and three np orbitals can accommodate only two electrons. 1 When there are an odd number of valence electrons 2 When there are too few.
Write the Lewis structure for each molecule. Write a Lewis structure that obeys the octet rule for each molecule or io 0505. And that is EIGHT and the octet rule is satisfied.

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Bf4 Lewis Structure How To Draw The Lewis Structure For Bf4 Youtube

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Youtube

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization