Pcl3 Resonance Structures
The Lewis dot structure. What is the formal charge of this sulfur atom.
Is Pcl3 Non Polar Or Polar Why Quora
Only the electrons move not the atoms.

Pcl3 resonance structures. Hree pairs will be used in the chemical bonds between the P and Cl. Indicate the type of hybrid orbitals used by the central atom in PCl3. CN H H H N H C N H H N H H CN H H.
Select the most Important resonance structure for this species based on the formal charges on the atoms of the three. The actual bonding in such molecules however is thought to be an average of the bonding present in all the resonance structures. Each of the three structures is a resonance contributor.
In the PCl 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. They differ only in that a pair of π electrons has moved onto the oxygen atom. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
Two or more resonance structures are said to exhibit resonance. Feel free to omit lone pairs and C-Hs or draw themyour choice Then below each resonance structure describe whether each would be a major or minor contributor. In 1 there is only resonance as only O-.
Instead we use the concept of resonance. The double-headed arrow implies a movement of the electrons and an actual shifting of the structure from one to another. The molecule is a resonance hybrid of the two structures.
Is ClO3 a resonance structure. So PCl3 does not exhibit resonance. If two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion the actual distribution of electrons is an average of that shown by the various Lewis structures.
So this molecule is polar. Draw three resonance structures for CS2. Thus if the conjugate base is stablereaction moves forward and H generated will be more thus more is the acidic strength.
If you can do those Lewis structures PCl 5 will be easy. EqPCl_3 eq Resonance Structures. Isomers differ in the location of atoms.
OCl2 does not have resonance structures and does not exhibit resonance. Resonance structures differ in the location of electrons. In the resonance structure of sulfur dioxide the sulfur atom has one single bind and one double bond.
Sp3 Molecular geometry of PCl3 is trigonal pyramidal with asymmetric charge distribution on central atom. Resonance structures as there are boxes to put them in. To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets.
Resonance structures are possible when multiple structures can be used to represent the structure of a molecule or ion. This is better explained through the structures-Now it is a fact that if the product is stable the reaction will move forward. For example acetone has two resonance contributors.
This species has its three atoms bonded sequentially in the following fashion. Draw your resonance structures so that the atoms in them are bonded together in this order. O2 has a double bond in its normal form.
Is O3 resonance or no resonance. Resonance structures represent the same compound. Answer to PCl3 ICl3 NO2 IF5 XeF2 IBr4 - SF6 Lewis Structure 3-D Sketch Are There Resonance Structures.
Finally draw a resonance hybrid that illustrates partial charges and multiple bonds. Yes the chlorate ion has three major contributors to the resonance hybrid. Both structures account for the needed 18 valence electrons 6 from 3 bonds and 12 as lone pairs placed on the oxygen atoms.
PCl 3 is similar to PBr 3 and PF 3. Does O2 have resonance. The actual distribution of electrons in each of the nitrogen-oxygen bonds in latextextNO_2.
Ozone or O3 has two major resonance structures that contribute equally to the overall hybrid structure of the molecule.

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

What Is The Molecular Geometry Of Pcl3 Study Com

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

How To Calculate The Formal Charges For Pcl3 Phosphorous Trichloride Youtube

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Is The Electron Dot Structure Of Pcl3 Determined Quora

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
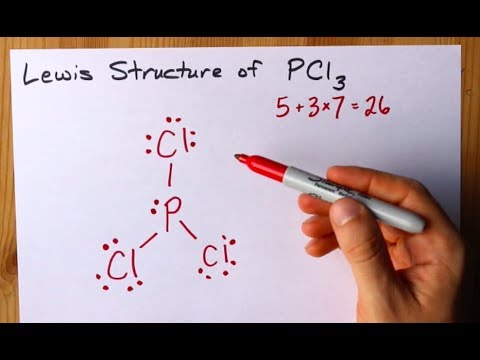
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Pcl3 Phosphorus Trichloride Lewis Structure

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
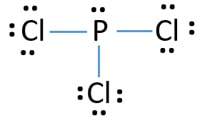
Pcl3 Phosphorus Trichloride Lewis Structure
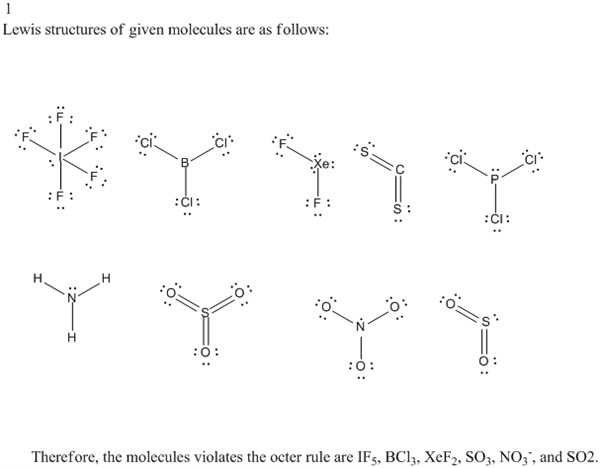
1 Look Again At The Lewis Structures That You Drew Chegg Com
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization


