Phosphorus Pentafluoride Lewis Structure
Lewis Dot Structure for PF5 PF 5 Name. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot.
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
It is mainly used to make phosphate esters such as tricresyl phosphate Exposure to a high concentration of PCl5 can lead to serious health problems.
Phosphorus pentafluoride lewis structure. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. Expert Answer 100 11 ratings Previous question Next question Transcribed Image Text from this Question. Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs.
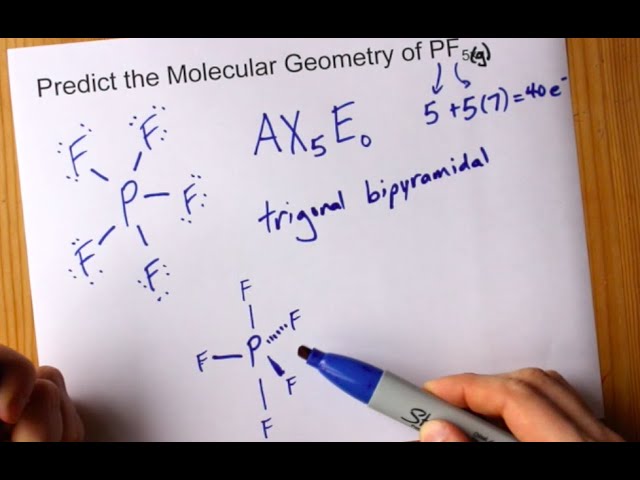
Phosphorus Pentafluoride PF 5 Lewis and Three-Dimensional Structures Trigonal Bipyramid 3-Dimensional View of PF 5 Chemistry Home Dr. Draw a Lewis structure for PF5. P - F ideal Bond Angle In The Structure.
Sulfur hexafluoride or SF6 is an inorganic greenhouse gas. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. PF5 Lewis StructureLewis Structure of PF5 Phosphorus PentafluorideDraw Lewis Structure for PF5PF5LewisStructure.
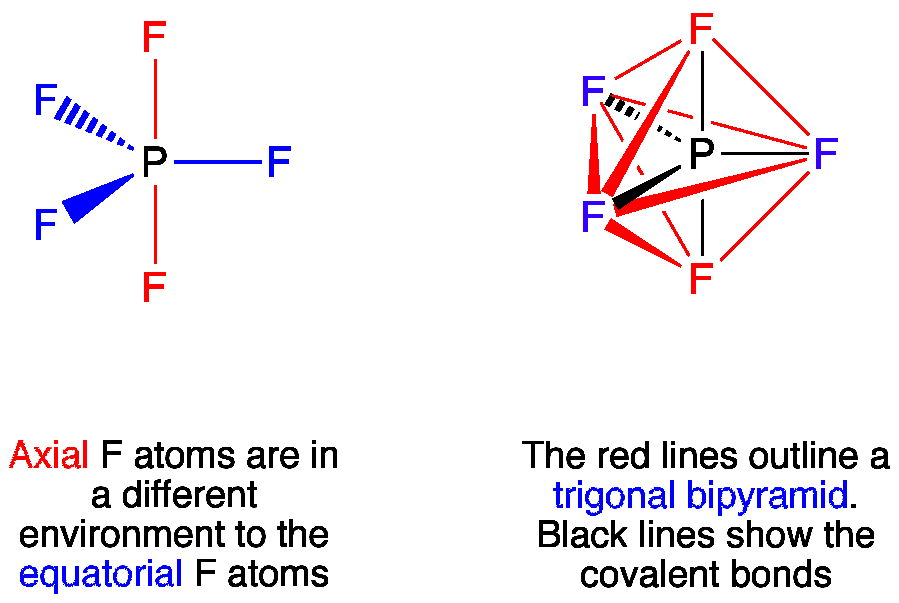
Sundin Home sundinuwplattedu. Thus it has two distinct types of PF bonds axial and equatorial. Phosphorus pentachloride lewis structure 01102021 0 Comments in Uncategorized by.
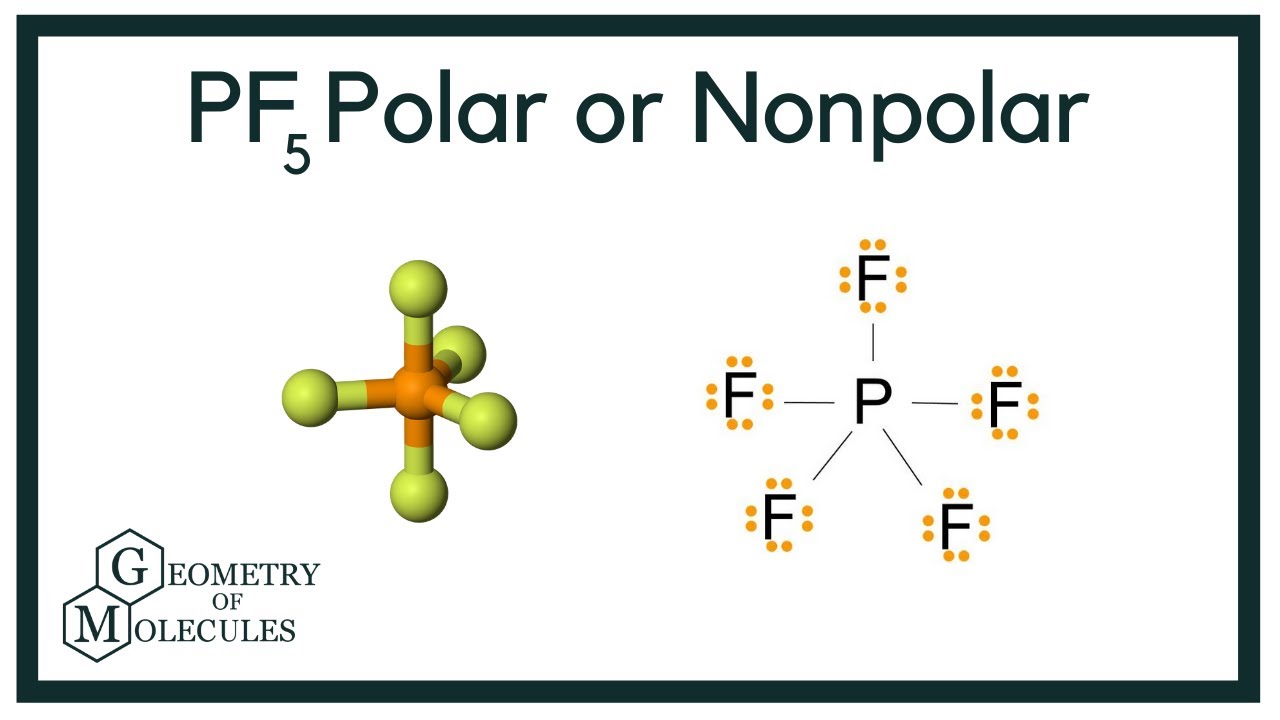
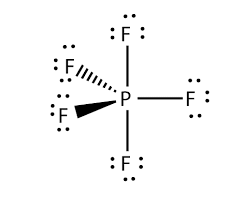
The Phosphorus Pentafluoride PF5 is a nonpolar molecule because the configuration of PF5 has trigonal bipyramidal and PF5 consists of a central phosphorus atom surrounded by five fluorine atoms. One electron is shared by each fluorine atom to complete its octet. Then draw the 3D molecular structure using VSEPR rules.
Draw A Lewis Structure For PF5. What Is The Smallest F. Facebook Twitter Pinterest linkedin Telegram.
Phosphorus pentachloride is the chemical compound with the formula PCl 5It is one of the most important phosphorus. By the reaction of FSO3H on fluoride and phosphate. I am struck by the ultra-covalent properties of this molecule.
Show transcribed image text. PF5 - Phosphorus Pentafluoride. One s orbital three p orbitals.
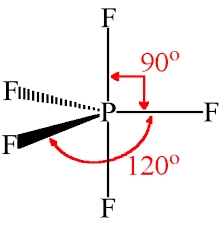
40 Phosphorus pentafluoride is a trigonal bipyramidal molecule. This is called a degeneracy and it turns out that nature tends to pick both neither and a combination of the two bonds. The length of an axial PF bond is distinct.
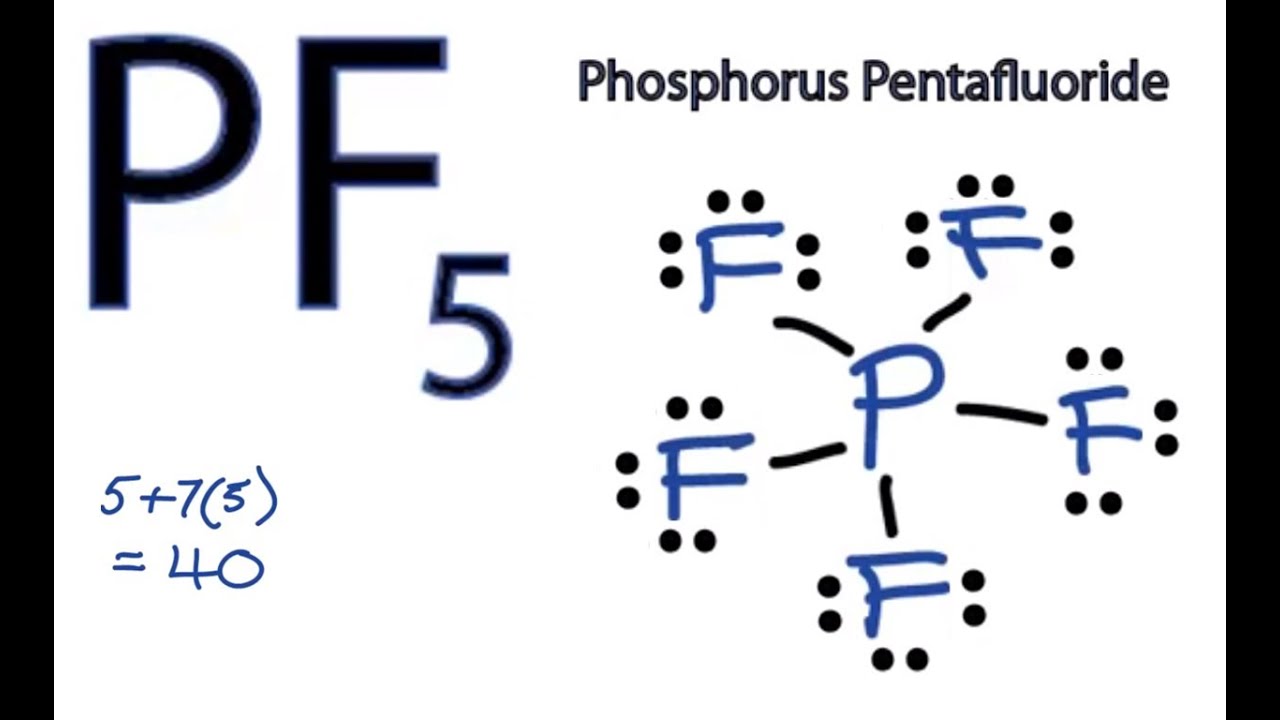
In PF5 the Phosphorus atom contains a total of 5 valence electrons and fluorine has 7 electrons in its outermost shell. The oxidation number of phosphorus in phosphorus pentafluoride is 5. Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method.
I am not a doctor. Phosphorus pentafluoride Molecular structure. Sncl3 Lewis Structure What is the molecular geometry of sncl3.
Trigonal bipyramidal State at room temperature. Phosphorus pentafluoride was first prepared in 1876 through fluorination of phosphorus pentachloride using arsenic trifluorideOther routes to PF5 have included fluorination of PCl5 by HF AgF benzoyl fluoride SbF3 PbF2 or CaF2It can also be made by the reaction of PF3 and fluorine chlorine or chlorine in contact with calcium fluoride. Therefore this molecule is nonpolar.
Start typing to see posts you are looking for. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. There are no lone pairs in the Lewis Structure of PF 5 and there are five single bonds between Phosphorus and Fluorine atoms.
3 PCl 5 5 AsF 3 3 PF 5 5 AsCl 3 Structure. So the three planar fluorines electronegativity cancels. The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3 but when it is in an excited state the electrons from 3s orbital get unpaired.
First draw the Lewis dot structure. It is a colourless toxic gas that fumes in air. SOLUTION a The Lewis structure of the SnCl3 ion looks like this.
There are five half-filled orbitals. All five fluoride elements are attracted to the phosphorus element in the center and have eight electrons in their valence shells. Phosphorus pentafluoride PF 5 is a phosphorus halide.
Sundin Home sundinuwplattedu Chemistry Home Dr. This problem has been solved. Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method.
Hybridization of Phosphorus Pentafluoride PF5 If we see the lewis structure of this molecule. 55 kg m -3 gas The following are some synonyms of phosphorus pentafluoride. In phosphorus pentachloride the central phosphorus atom makes five single bonds to chlorine atoms and as a result has ten electrons.

Pcl6 Lewis Structure How To Draw The Lewis Structure For Pcl6 Youtube

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure Lewis Structure Of Pf5 Phosphorus Pentafluoride Draw Lewis Structure For Pf5 Youtube
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride Youtube

The Science Of Studying Lewis Dot Structure For Pf5

Pf5 Molecular Geometry Shape And Bond Angles Youtube

Clf5 Lewis Structure How To Draw The Lewis Structure For Clf5 Chlorine Pentafluoride Youtube

The Science Of Studying Lewis Dot Structure For Pf5

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
Is Pf5 Polar Or Nonpolar Check Phosphorus Pentafluoride Polarity Geometry Of Molecules

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Pbr5 Molecular Geometry Lewis Structure Shape Bond Angle And More

Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube
Vsepr Pf5 Phosphorus Pentafluoride