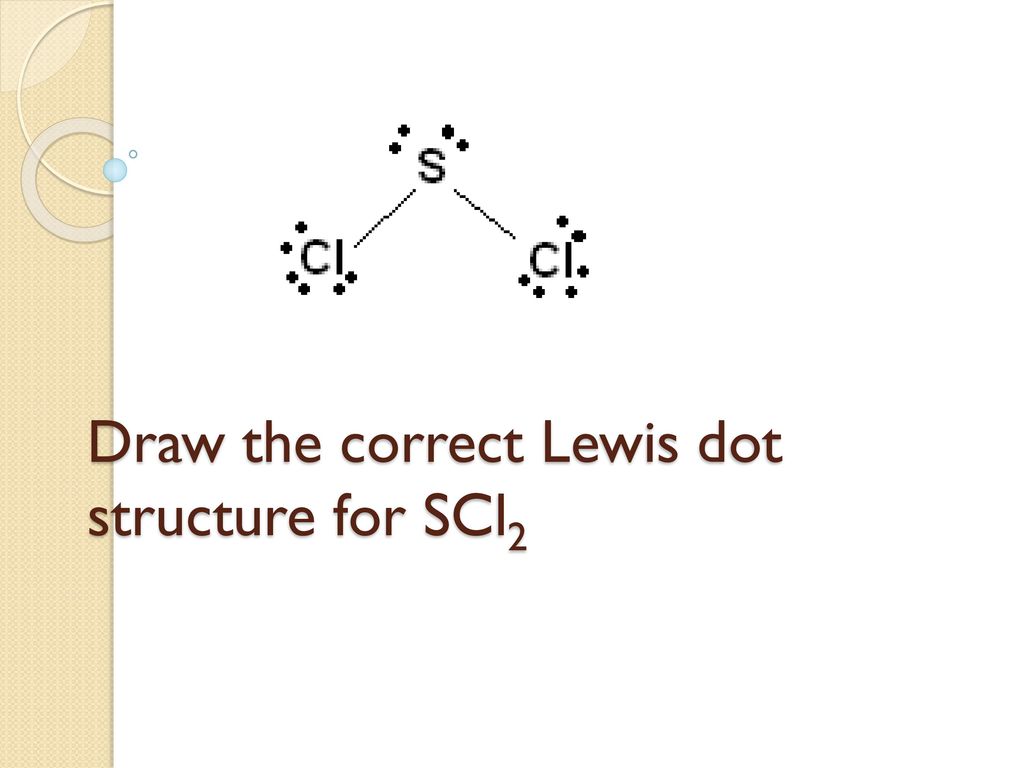
Scl2 Lewis Structure Shape
Draw the Lewis structure for SCl 2 and use it to identify the correct statements that follow. Electronic Shape Of SCl2.

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
In this article we will study Chlorine gas Cl2 lewis dot structure its molecular geometry is it polar or non-polar hybridization etc.
Scl2 lewis structure shape. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Notice that SCl2 has a molecualr geometry that is very similar to waters the only differences being the smaller bond angle water has a bond angle of 10445 and the longer bond lenght water has a bond lenght of 9584 pm. A three-step approach for drawing the HBr Lewis structure can be used.
Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. In this post we discussed the method to construct the CH3I Lewis structure. Facebook Twitter Pinterest linkedin Telegram.
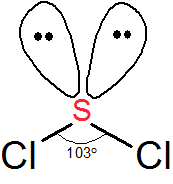
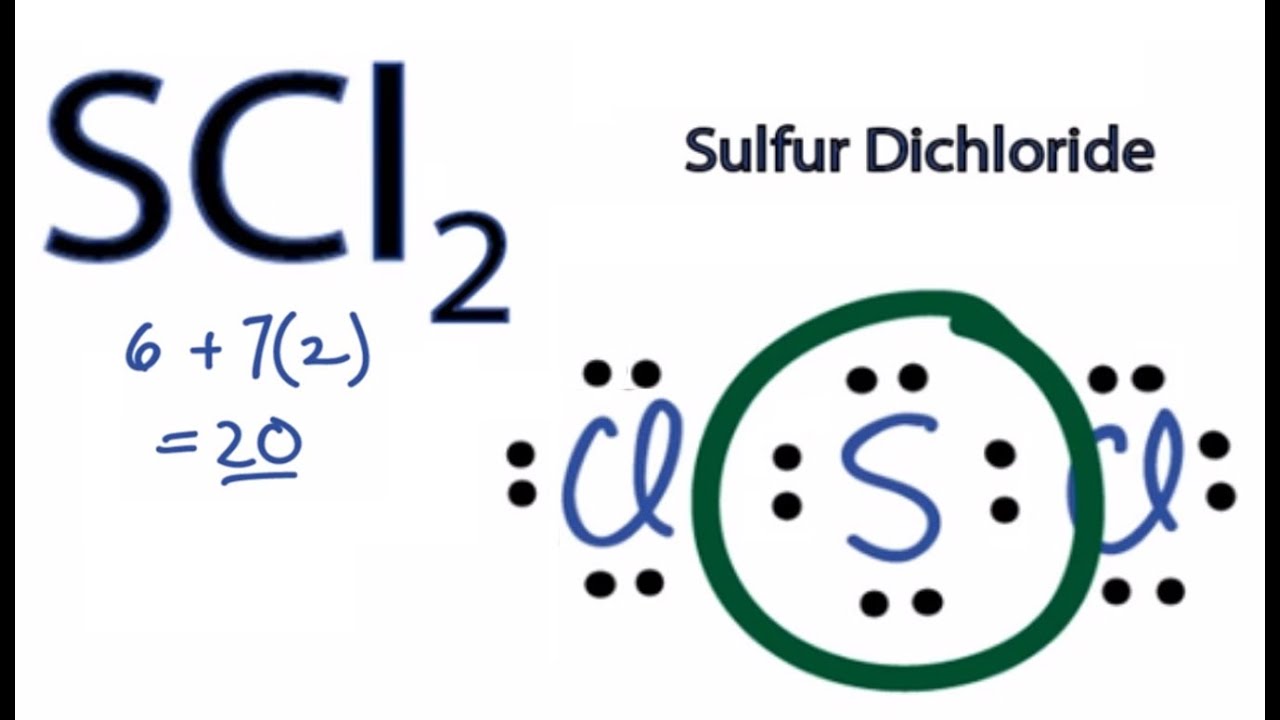
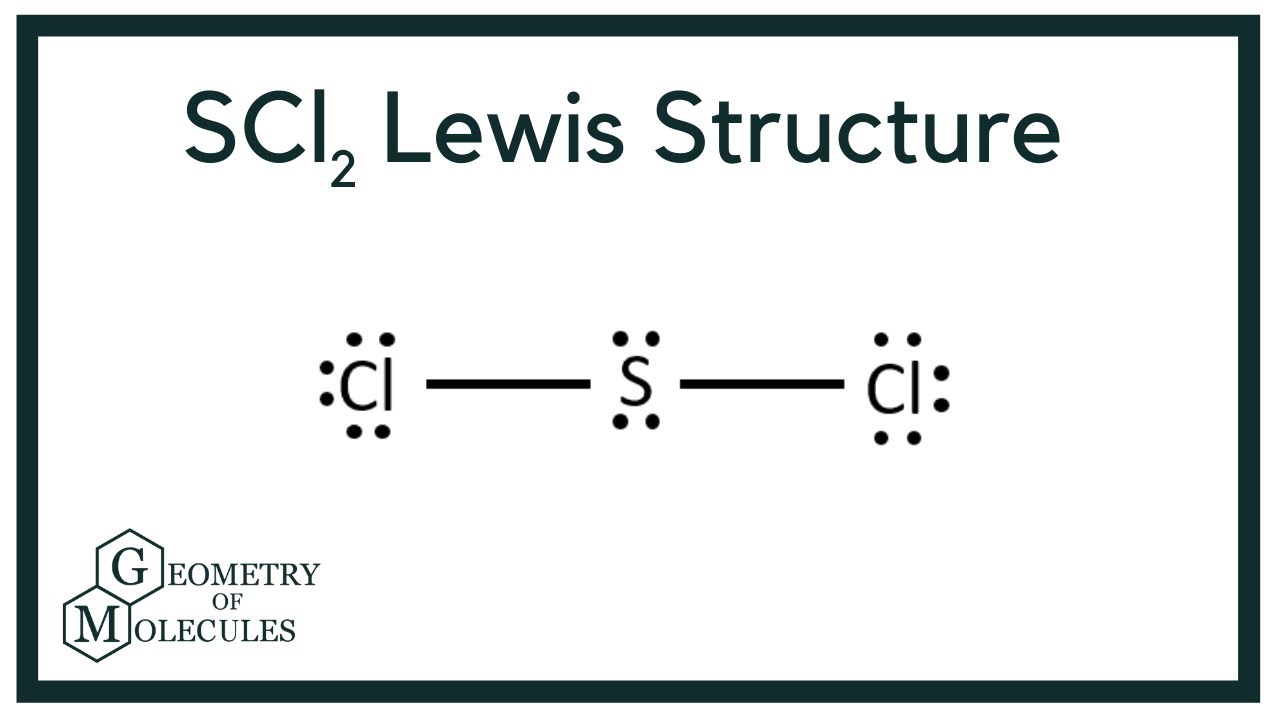
The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. SCl2 Molecular Geometry and Shape. Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center.
The molecular geometry shape is linear. The molecular geometry of SCl2 is simply determined by considering only the number of bonded atoms and as. The first step is to sketch the Lewis structure of the HBr molecule to add valence electrons around the bromine atom.
The electronic shape of SCl2 is tetrahedral and four electrons surround sulfur. 19 Jul CF4 Lewis Structure Molecular Geometry Hybridization Bond Angle and Shape. Facebook Twitter Pinterest linkedin Telegram.
It appears as yellow-green gas at room temperature and pressure. Key Points To Consider When Drawing The HBr Electron Dot Structure. First the valence electrons are placed around the carbon atom.
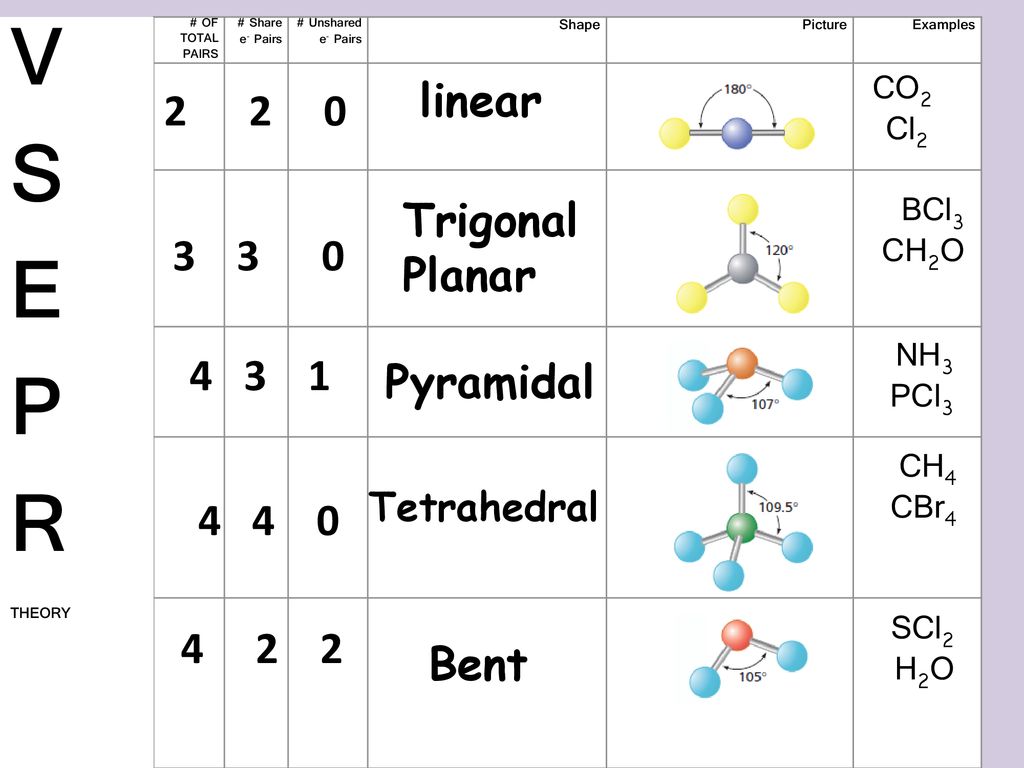
As seen in the Lewis structure above the Chlorine atoms repel each other. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. Sulfur is the central atom in the Lewis structure of SCl2 that has a steric number equal to 4 hence the electron geometry of SCl2 is tetrahedral.
Is SCl2 molecule polar or nonpolar. SOCl2 is pyramidal in shape this is because sulphur being the central atom has 6 electrons in its valence orbit out of that six electrons 2 are is S orbital and the rest are in P orbital. Electron geometry which is determined by the steric number will be tetrahedral while molecular geometry which is determined by the coordination number will be bent.
The molecule is nonpolar. Thus the hybridization of SCl2 is sp3. The second step is to add valence electrons to the one hydrogen atom and the final step is to combine the step1 and step2 to get the HBr.
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. To calculate the formal charge on the central sulfur atom of the SBr2 molecule by using the following formula. Second place the valence electron on the iodine and hydrogen atoms.
Is SCl2 molecule polar. However upon the addition of the two lone pairs on Sulfur the molecular geometry becomes bent. Chlorine gas is very reactive thus they bonded to each other to stabilize the molecule.
SCl2 Lewis Structure Molecular Geometry Hybridization Bond Angle and Shape. Two of them are lone pairs and the remaining two are bond pairs. This gives us a linear shape initially.
This gives SCl2 a bond angle of 103. None of these require pi-bonding which is the method of formation for double and triple bonds. SBr2 Lewis dot structure.
Select all that apply. This is in accordance with the VSEPR theory. The formal charge on the sulfur atom of SBr2 molecule V.
Chemistry QA Library Draw the Lewis electron dot structure for SCl2 and discuss its molecular geometry.
Valence Shell Electron Pair Repulsion

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity
Types Of Bonding And Lewis Structures Ppt Download

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube
Scl2 Lewis Structure Sulfur Dichloride Youtube

What Is The Molecular Shape Of Scl2

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

What Is The Electron Pair Geometry And Mol Clutch Prep
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

Is Scl2 Polar Or Nonpolar Techiescientist
Bond Angle Of Scl2 Lewis Structures