Sif4 Lewis Base
Silicon is larger than carbon and better able to stabilise 4e3c bonds due to its lower electronegativity. Typische Lewis-Basen sind zB.
Chem Molecular Shape Molecular Geometry Scientific Tutor
Amphoteric-usually talking about Bronsted-Lowry so think about it as compounds that can either donate or accept H.
Sif4 lewis base. Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors and thus are classified as Lewis acids eg SiBr4 SiF4. On the other hand such process is not possible for compounds formed by 2nd period elements. Search More info Main menu.
None of the above is a Lewis acid. CF4 can not act as Lewis acid. Depending on the nature of ceX- the pentacoordinated ceSiF4X- species may either break down under liberation of either ceF- mathrmS_NSi substitution or ceX-.
What is the advantage of using the lewis acidbase system. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. Let us help you simplify your studying.
Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg. Can Oh function as a Lewis base. Find more Chemistry widgets in.
A Lewis base is any species that donates a pair of electrons to a Lewis acid. Is sicl4 polar or nonpolar. C The overall charge of the molecular species.
What do we call compounds that. Fastest Reliable Cheapest Game Keys and Digital Services at DamnModz. Can OH- function as a Lewis base.
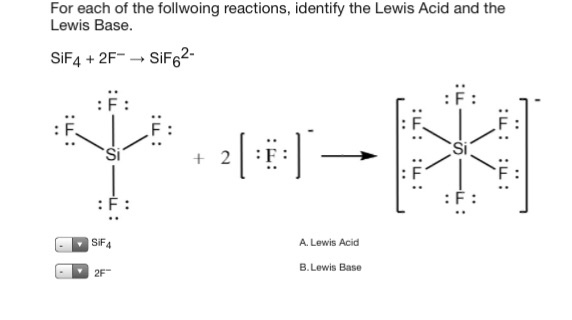
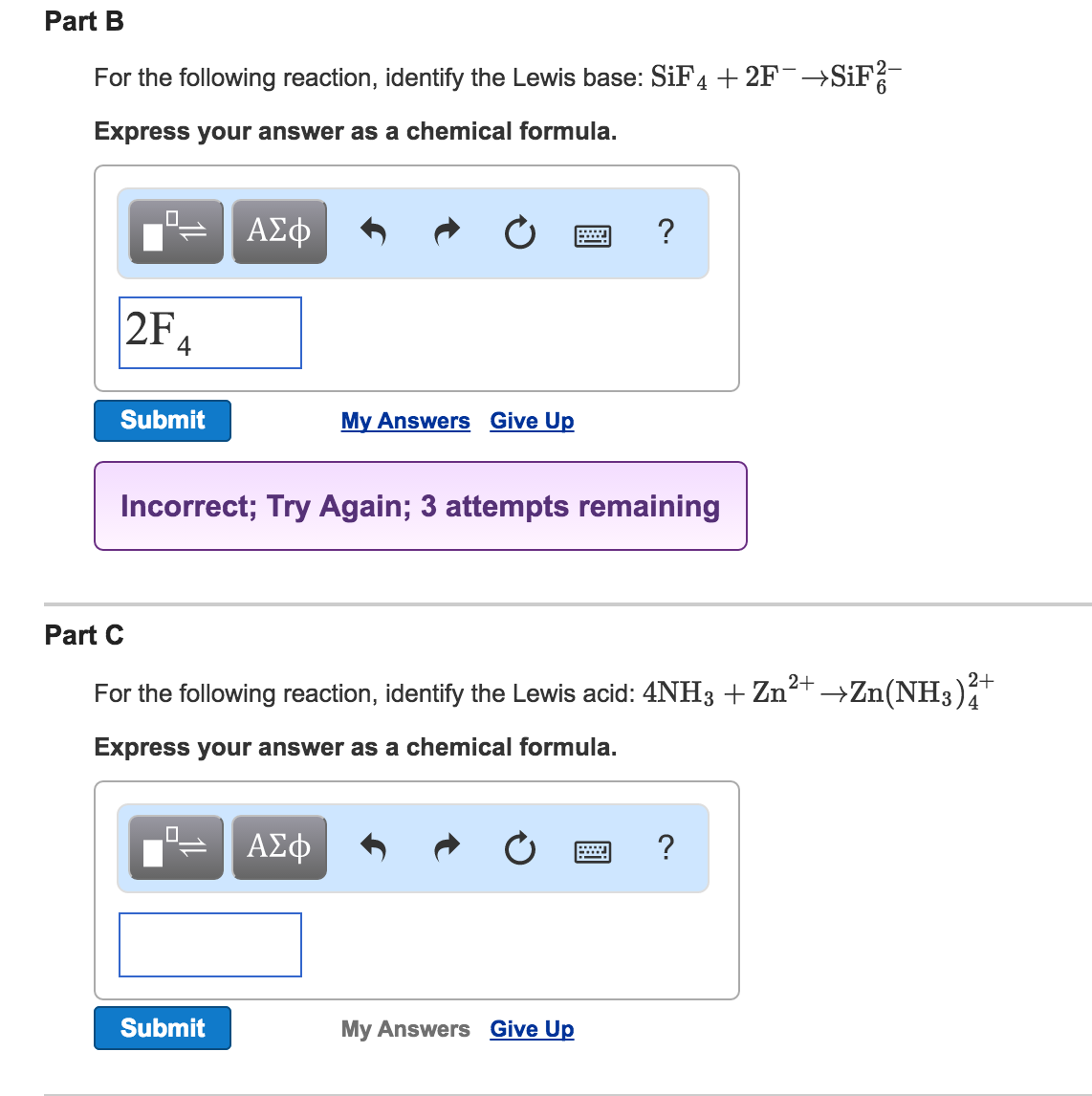
Please Choose Either A Or B. F- to give SiF6 2- ion. A Lewis base is a chemical compound that can donate a pair of electrons to a suitable electron-pair acceptor Lewis acid to form a Lewis adduct.
Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. SiF4 2F- SiF62- SF4 F- SF5- Its taking that negative charge electrons from the F-Be careful not to confuses these with the Bronsted-Lowery acids. NH 3 H 2 O F- CN-oder CO.
Lewis-Basen sind Atome oder Moleküle die ein freies Elektronenpaar besitzen das zur Ausbildung einer kovalenten Bindung Atombindung geeignet ist. Learn this topic by watching Lewis Acid and Base Concept Videos All Chemistry Practice Problems Lewis Acid and Base Practice Problems. Compounds that can act as both an acid and a base.
Is SiF 4 a lewis base or lewis acid. Similarly Lewis acids can be classified as. Cyanide is isolectronic with CO and while not as pi-.
Its also a pi acceptor Lewis acid. BF3 AIF3 SiF4 and PF5 all contain electron deficient inner atoms. Im Gegensatz zur Lewis-Säure ist eine Lewis-Base ein Elektronenpaardonator.
F- to give SiF6 2- ion. Abstract Lewis acidbase interactions between SiF 4 and a wide range of molecular negative ions are reported here for the first time. For example it reacts with F- to form SiF_62- hexafluoridosilicate2-.
Is cf4 lewis base. Hence they can not act as Lewis Acid eg. Thus 4e3c bonds are sufficiently accessable and stable for silicon in ceSiF4 to act as a Lewis acid.
Lewis acid can accept a pair of electron from Lewis base. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg. The most common Lewis bases are ammonia alkyl amines and other conventional amines.
SiF_4 can act as a Lewis acid because Si can expand its octet. Heres how SiF4 acts like a Lewis acid. Indeed the required interaction would be the Lewis bases lone pair with sigma_ceC-F an orbital which is already well hidden by the three other.
Lewis acid can accept a pair of electron from Lewis base. A Lewis acid is an electron-pair acceptor. From chemwikiucdavisedu SiF_4 can accept electron pairs and expand its octet to 12.
Commonly Lewis bases are anionic in nature and their base strength generally depends on the pK a of the corresponding parent acid. Oh- donates a pair of its electrons. Hence they can not act as Lewis.
From measurements performed by pulsed electron-beam. SARL K FOOD COMPANY. The molecular anions include those formed by simple electron attachment to p -benzoquinone benzophenone nitrobenzene and 21 substituted nitrobenzenes and also include the o - and p -nitrophenoxy anions.
On the other hand such process is not possible for compounds formed by 2nd period elements. Since Lewis bases are electron-rich species that have the ability to donate electron-pairs they can be classified as nucleophiles. The fluorides BF3 AIF3 SIF4 and PF5 are Lewis acids.
Electron pair donor-OH- CN- NH3. We can recognize acids that do NOT have H because they accept electrons.

Lewis Acid And Base Concept For Each Of The Following Chegg Com
Https Www Neetprep Com Subject U3viamvjddo1na Topic Vg9wawm6njq4 Doubt Rg91ynq6mzk4njy

Which Are Of The Following Are Lewis Acids Cheek Chegg Com

Is Sif4 A Polar Or A Non Polar Molecule Quora

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora

Ch4 Acids And Bases Ppt Video Online Download
Why Does Sif4 Act As A Lewis Acid Example

Is Sif4 A Lewis Base Or Lewis Acid

Solution Which Of The Following Is A Lew Chemistry
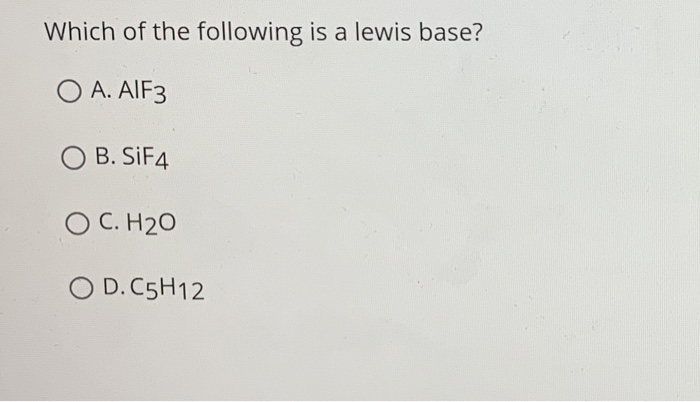
Which Of The Following Is A Lewis Base O A Alf3 O Chegg Com
For Each Of The Following Reactions Identify The Chegg Com
Which Are Of The Following Are Lewis Acids Cheek Chegg Com
Part B For The Following Reaction Identify The Lewis Chegg Com

Is Sif4 A Lewis Base Or Lewis Acid
What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora
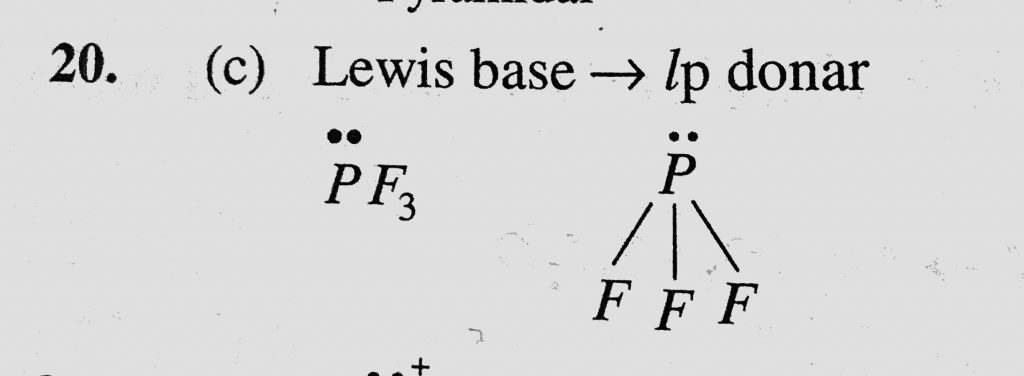
Which Of The Following Fluoro Compounds Is Most Likely To Behave As A Lewis Base A Sif4 B Bf3 C Pf3 D Cf4 Sahay Lms
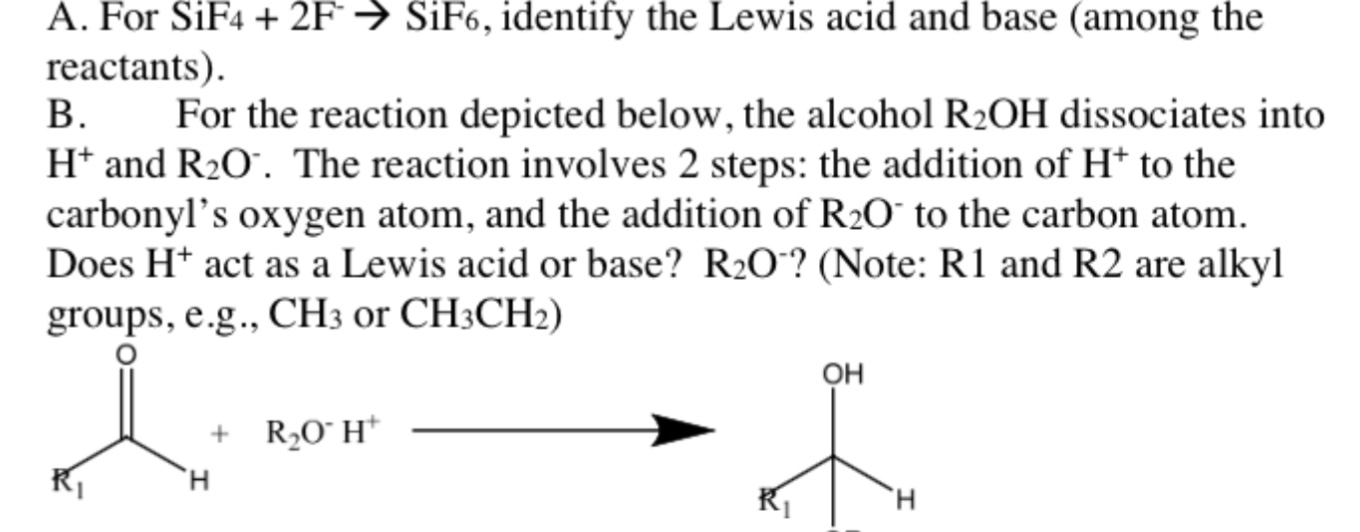
A For Sif4 2f Sif6 Identify The Lewis Acid And Chegg Com