So3 2- Lewis Structure Lone Pairs
The electrical dipole moment of gaseous sulfur trioxide is zero. Molecule of Valence e- Lewis Structure e- Domains 1-6 Bonded Atoms Lone Pairs Electronic Geometry Molecular Geometry DrawName Polar or Nonpolar PNP H2S CF4 CH2Cl2 HOCN PH3 SO2 Molecule of Valence e- Lewis Structure e- Domains 1-6 Bonded Atoms Lone Pairs Electronic Geometry Molecular Geometry DrawName.

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora
In this case the steric number is 3 so the hybridization of SO 3 is SP2.
So3 2- lewis structure lone pairs. In Lewis Structure of N 2 O 3 one oxygen atom and nitrogen atom has -1 and 1 charges respectively. Are SO3 and PCl3 isostructural. Incorrect sulfite ionpng However when you delocalize the oxygen lone pairs and form a double bond the charge is decreased so the molecule becomes more stable.
So why is it an acid and not a base. But there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. You do not have the required permissions to view the files attached to this post.
SO3 is isostructural with BCl3 both have trigonal planar structure. Clutch really helped me by reinforcing the things I learned in class and making exam reviews a breeze. When you draw the ion with all single bonds and 3 lone pairs on each oxygen there is a formal charge of 1 on S and -1 on each O as shown.
Dinitrogen trioxide is a one of the oxides of nitrogensThere are three oxygen atoms around two nitrogen atoms. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO32- ion. Here is the structure build with my tools for SolidWorks.
Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO32- ion. Lewis Structure of N 2 O 3 Dinitrogen trioxide. In terms of electron-counting formalism the sulfur atom has an oxidation state of 6 and a formal charge of 2.
In the best Lewis structure of OC12 around the oxygen there are and 5 points O 6 bonding pairs and 2 lone pairs 2 bonding pairs and 8 lone pairs 4 bonding pairs and 0 lone pairs O2 bonding pairs and 2 lone pairs The formula of compound formed between Ammonium ion NH and Sulfite ion SO32- is. This is an unusual molecules in the Sulfur S is normally -2 but in this case is 6 as 3 double bonds at the equatorial positions to each of the 3 -2 negative valance Oxygens. What makes H2SO3 lose H ions but not attract them.
Since S is a third row element can accommodate more than 8 valence electrons. So H2SO3 is an acid but the lewis structure which I inserted below has lone pairs to attract H ions from H2O which will make OH- ions. I also go over hybridization shape and bond angles.
There are 3 sigma bonds which mean we have three regions. I quickly take you through how to draw the Lewis Structure of SO3 2- Sulfite Ion. The central carbon atom undergoes octet stability.
Therefore five electron groups are around the central atom of SO32- ion. What is the Lewis dot structure of SO3 2. We can find out the structure of molecule with the help of this structure.
There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Sulfur brings 6 and oxygen brings 3 each. There is more equal charge distribution which further stabilizes the structure.
Complete the middle carbon atom stability and if necessary apply a covalent bond. NH42 SO32 A B. Therefore the structure in Step 1 is a plausible Lewis structure of SO 3-2.
Electrons are placed around each atom so that the octet rule is obeyed. How is SO3 2 formed. Our mission is to help you succeed in your Chemistry class.
The formula of steric number is the addition of the number of atoms bonded and lone pairs of electrons. Therefore five electron groups are around the central atom of SO32- ion. Lewis structure provides the bonding present between atoms of a molecule and lone pair of electrons involved in a molecule.
What is the Lewis dot structure of SO3. Now we are going to learn how to draw this lewis structure. No lone pair of electrons on the carbon atom in the tetrahedral geometry of the CH3I molecule.
So there are not lone pairs but double bonds. Central atom of SO32- ion is sulfur. So far weve used 14 of the CH3I Lewis structures total 14 outermost valence shell electrons.
One sigma bond and two pairs of unbonded electrons. That means we have an S and two P orbitals to be hybridized. Therefore P 6n 2 V 6 4 2 26 0 There are no π electrons in SO3-2.
In the Lewis dot structure of sulfite ion SO32- how many lone pairs of electrons are present on the sulfur atom. Central atom of SO32- ion is sulfur. Transcribed image text.
Each oxygen atom has two lone pairs in SO 3 lewis structure. The Lewis structure consists of an SO double bond and two SO dative bonds without utilizing d-orbitals. Hybridization of SO 3 molecule.

How Many Electron Dots Are In The Lewis Structure Of So 3 2 Study Com
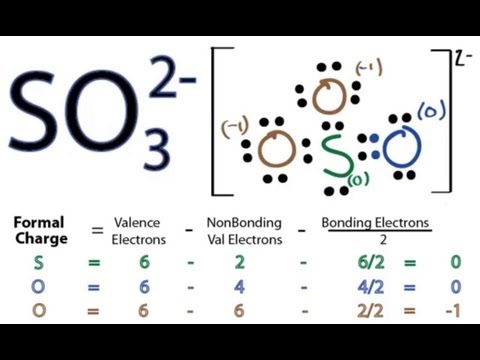
So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora
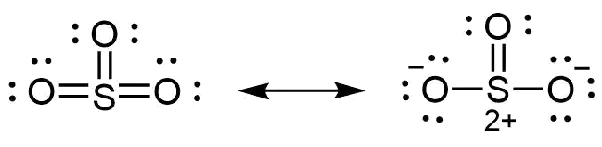
What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

So3 2 Molecular Geometry Shape And Bond Angles Youtube

Lewis Structure For So32 Sulfite Ion Resonance Structures
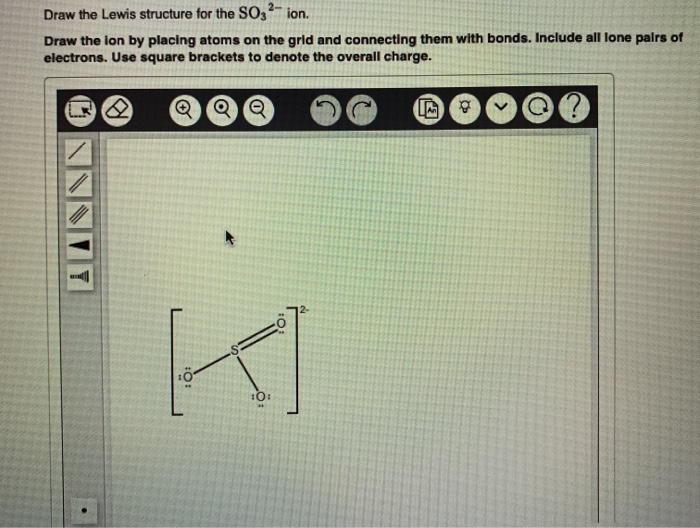
2 Draw The Lewis Structure For The So32 Ion Draw Chegg Com
How Is The Hybridization Of So3 2 Determined Quora

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube
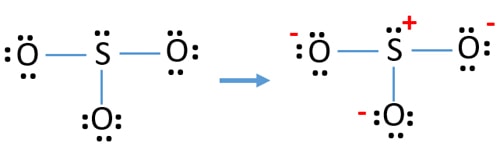
Lewis Structure For So32 Sulfite Ion Resonance Structures

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora
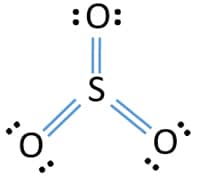
Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps
.jpg)
Lewis Structure For So32 Sulfite Ion Resonance Structures
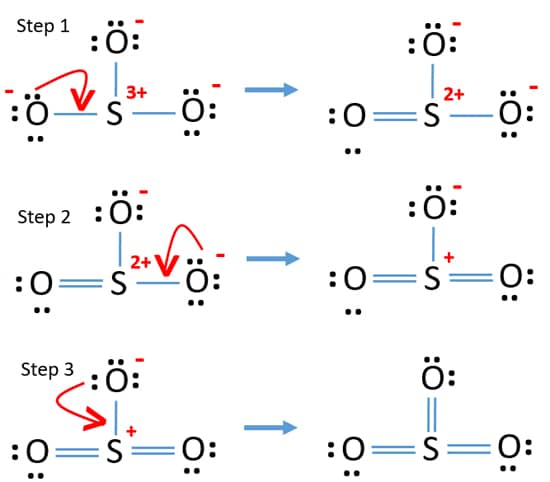
Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps

Draw The Lewis Structure For The So32 Clutch Prep
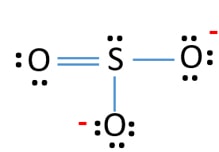
Lewis Structure For So32 Sulfite Ion Resonance Structures