Xef4 Lewis Structure
This chemical compound is formed when xenon reacts with fluorine. You can see in this molecules the central atoms is xe and four fluoride atoms are attached with central atoms.

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas
Bromine gets 12 electrons in order to make 5 bonds with surrounding atoms.

Xef4 lewis structure. The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and Fluoride atoms. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. This electron arrangement is known as Trigonal Bipyramidal.
The valance electrons. Its chemical equation could simply be written as. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero.
Lewis structure of xef4. Square Planar 3-Dimensional View of XeF 4 Chemistry Home Dr. Draw the Lewis structure for i CO ii XeF4 iii PCl3.
The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Find octet e- for each atom and add them together. Find valence e- for all atoms.
EPUB How To Draw The Lewis Dot Structure For Xef4 Inorganic Chemistry-Gary Wulfsberg 2000-03-16 Both elementary inorganic reaction chemistry and more advanced inorganic theories are presented in this one textbook while showing the relationships between the two. Write the Lewis structure for XeF4. See full answer below.
Xe has 8 ve 4 F atoms have a total of 28 so there are 36 total ve in the molecule. SOLUTION a The Lewis structure of the SnCl3 ion looks like this. Xe 2 F2 - XeF4.
Because the valence electrons of both atoms is. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. As with all Lewis structures you first count the valence electrons then distribute them in pairs forming octets and allowing for the ability of Xe to accommodate an expanded octet.
The Lewis structure for XeF4 has a total of 36 valence electrons. Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. CBr2H2 BH3 XeCl4 SF4 HCl.
But the overall molecules xef4 have 36 valance electrons. Sundin Home sundinuwplatt. Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1.
In this process elemental fluorine supposedly oxidizes xenon under some specific conditions of temperature. Xe 2F2 XeF4. Does SeF4 obey octet rule.
It is a type of noble gas having the chemical equation of. Xenon Tetrafluoride XeF 4 Lewis and Three-Dimensional Structures. First the valence electrons are placed around the carbon atom.
Identify the geometry of XeCl4. The XeF4 has a solid white appearance and has a density of 4040 g cm3 in a solid form. The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair.
4 Xe-F single bonds use up 8 of these electrons and 3 non-bonding pairs of electrons complete each octet on the F atoms for a total of 24 more ve. How can the Lewis structure for XeF4 be determined. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table.
XeF4 is the chemical formula of the compound Xenon Tetrafluoride. In this tutorial we will learn how to draw lewis structure of XeF 4 step by step. Include all lone pairs of electrons.
Sncl3 Lewis Structure What is the molecular geometry of sncl3. In XeF 4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom. Draw the molecule by placing atoms on the canvas and connecting them with bonds.
Each fluorine atom has three lone pairs. The shape is like a seesaw. Each fluorine atom has three lone pairs.
XeF4 Lewis Structure Molecular Geometry Hybridization and MO Diagram. Second place the valence electron on the iodine and hydrogen atoms. In this post we discussed the method to construct the CH3I Lewis structure.
It is the worlds first binary compound discovered. Lewis structures are to represent the arrangement of valence electrons around atoms in a molecule.

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Science Coverage Is Asf5 Polar Or Nonpolar In 2021 Molecular Geometry Molar Mass Octet Rule

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Xef4 Molecular Geometry Xenon Tetrafluoride Is A Chemical Compound With Chemical Formula Xef4 It Is Produced By Molecular Geometry Chemical Formula Geometry

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots

Science Coverage Is Cf2cl2 Polar Or Nonpolar Molecular Geometry Covalent Bonding Solubility

Science Coverage Is Asf3 Polar Or Nonpolar In 2021 Molecular Geometry Polar Octet Rule
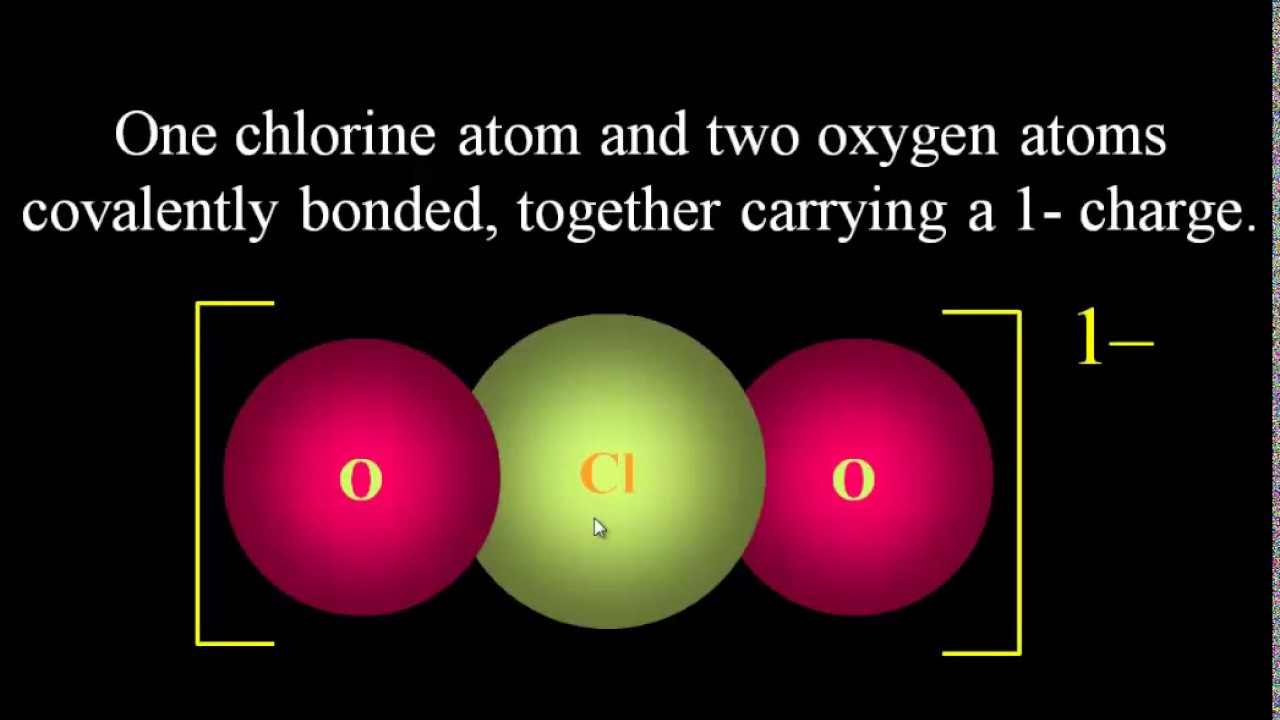
Polyatomic Ions Explained Origin Of Charge Drawing Polyatomic Ion Lewis Dot Structures Tutorial Youtube Polyatomic Ion Chemistry Class Physical Science

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Effect Of Loan Electron Pairs In Shaping H2o Molecule

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Hybridization Of Of2 Oxygen Difluoride In 2021 Molecules Oxygen Things To Come

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation