Does Xef4 Have A Dipole Moment
Dipole moment for a molecule is the vector sum of the individual bon dipoles. A ClF5 b 2ClO c 24TeCl d PCl3 e SeF4 f 2PH g XeF2.
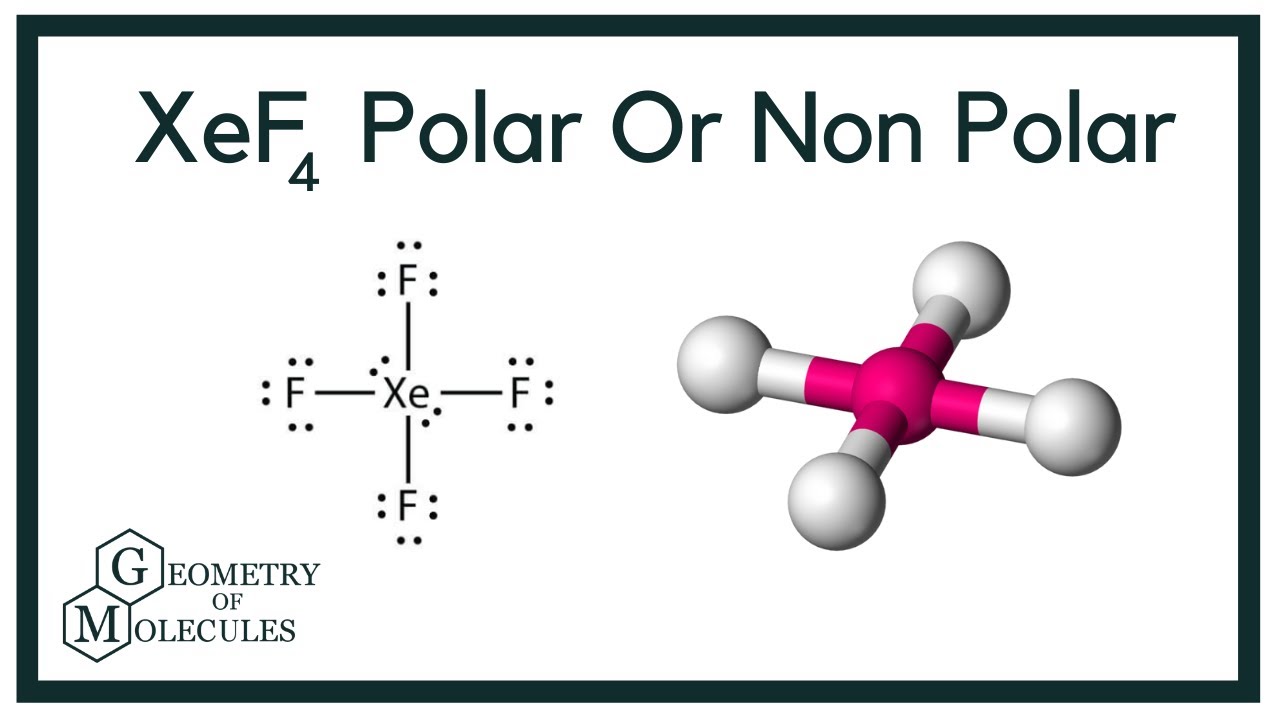
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
Since it is asymmetrical the molecule is polar and has a net dipole moment.
Does xef4 have a dipole moment. As you state the CF4 is symmetrical tetrahedral not planar so there is no net polar moment. In each case the individual bond dipoles cancel out leaving the molecules nonpolar. Therefore the S-F bond also gives a non zero dipole moment.
So SF4s geometry is seesaw. So CHF3 is expected to have greater dipole moment. I think you are mistaken for XeO4.
A molecule is said to be polar when it has a dipole moment creating partial positive and negative charges and forming unsymmetrical bonds. Does XeF4 have a tetrahedral shape. Is XeF2 dipole dipole.
Explain why CF 4 and Xef 4. So the hybridization will be sp3d2. Dipoles and Electrostatic Surfaces XeF 4 ClF 3 and CCl 3 Br.
Xef4Xenon Tetrafluoride Molecular Geometry Lewis. Is XeF4 Polar or Nonpolar. ICl4 I C l 4 molecule has 4 bond pairs and one lone pair which results in a see-saw structure.
Thus it is a neutral substance. Which of these molecules and ions have dipole moments. Chlorine trifluoride has three polarized bonds and they combine to produce a small molecular dipole along the Cl-F bond.
For TeF4 and ICl3 the arrangement of these molecules is such that the individual bond dipoles do not all cancel so each has an overall dipole moment is polar. Is o3 polar or nonpolar. 18 Feb February 18 2021.
For a molecule to have a dipole moment the individual dipole moments of its bonds must not cancel out. Why does XeF4 have no dipole moment. XeO3 has 3 dative bonds and a pair of lone pairThe vector sum of the 3 bonds will be 0 but the lone pair will add to the dipole moment so XeO3 will have a dipole moment but XeO4 wont.
Hence ICl4 I C l 4 is a polar molecule. A dipole moment means that theres a separation of charge in the molecule. Also due to the presence of lone pair would be distorted due to the electron dense cloud of the lone pairs.
Due to symmetry the dipole moment vector cancels out and thus XeF2 is a non-polar molecule. The dipole moment of this molecule is not zero. How to keep right color temperature if I edit photos with night light mode turned on.
Therefore XeF4 is a non-polar molecule or compound by nature. O3 is polar because. So the shape of XeF4 would be square planar rather than tetrahedral.
The dipole moment of the water molecule H2O is 6171030Cm. Does xef4 have a dipole moment. When we take the vector sum of these dipoles it adds up to be a net-zero Debye.
The XeF4 xenon tetrafluoride molecule is hypervalent with six electron pairs around the central xenon Xe atom. Someone please approve this answer. Is XeF4 an octahedral.
In XeF4 there are four Xe-F bonds each having an individual dipole moment with a magnitude and a direction. Owing to the symmetric arrangement of the Xe-F bonds and the non-bonding electron pairs the net effective dipole across the whole molecule is zero. Xef4Xenon Tetrafluoride Molecular Geometry Lewis.
Test bank for. Therefore there is no net dipole moment. The Xe-F bonds are all polarized but they cancel one another out so the molecule has no dipole.
Does xef4 have a dipole moment. A chlorine ion Cl of charge 1601019C is located at x300109m. Some of the examples of nonpolar molecules are Hexane NO2.
The shape of nonpolar molecules are symmetric. Hi I Need Someone To Complete The Following Probl. Does xef4 have a dipole moment.
There are twelve atoms around Xenon- eight from the four fluorine bonds two from each bond. Does h2o have a dipole moment. Does xef4 have a dipole moment February 18 2021 0 Comments 0 Comments.
XeO4 is tetrahedral and XeF4 is square planar and both are symmetrical. 0 3 D and H I 0. Is XeF4 polar or nonpolar.
The molecular geometry of XeF4 is square planar. Consider a water molecule located at the origin whose dipole moment p points in the x-direction. Assume that x is much larger than the separation d between the charges in the dipole so that the approximate.
The dipole moment of XeF2 is zero. The equation for dipole moment is as follows. Does xef4 have a permanent dipole moment Our tutors have indicated that to solve this problem you will need to apply the Dipole Moment concept.
Does TeF4 have a dipole moment. When two electrical charges of opposite sign and equal magnitude are separated by a distance an electric dipole is established. Explain why CF 4 and Xef 4 are nonpolar compounds have no net dipole moments while SF 4 is polar has a net dipo le moment.
Does xef4 have a dipole moment. Does Xef4 Have A Dipole Moment. The dipole moment of a molecule is therefore the.
Answer in Organic Chemistry for Blessing Akah 104754. All have polar bonds but only TeF4 and ICl3 have dipole moments. For example each of the two carbon-oxygen bonds in CO 2 has a dipole moment but the CO 2 molecule has no dipole moment because the dipole moments of the two carbon-oxygen bonds are identical in magnitude and opposite in direction resulting in a vector sum of zero.
Dip ole moment is. That one side. XeF4 is with tetrahedral shape where one side is a lone pair which the charge is imbalanced and it has dipole dipole attraction between molecules.
Does xebr4 have a dipole moment.
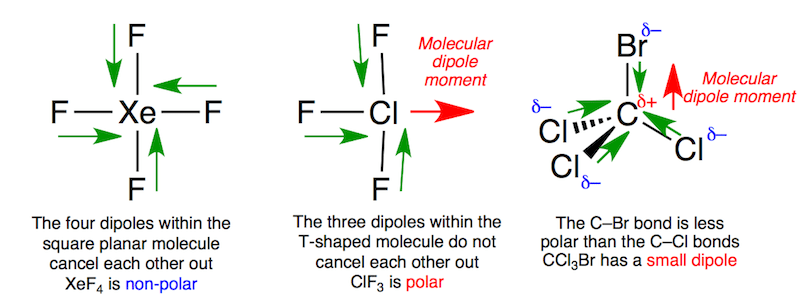
Dipoles And Electrostatic Surfaces Xef4 Clf3 And Ccl3br
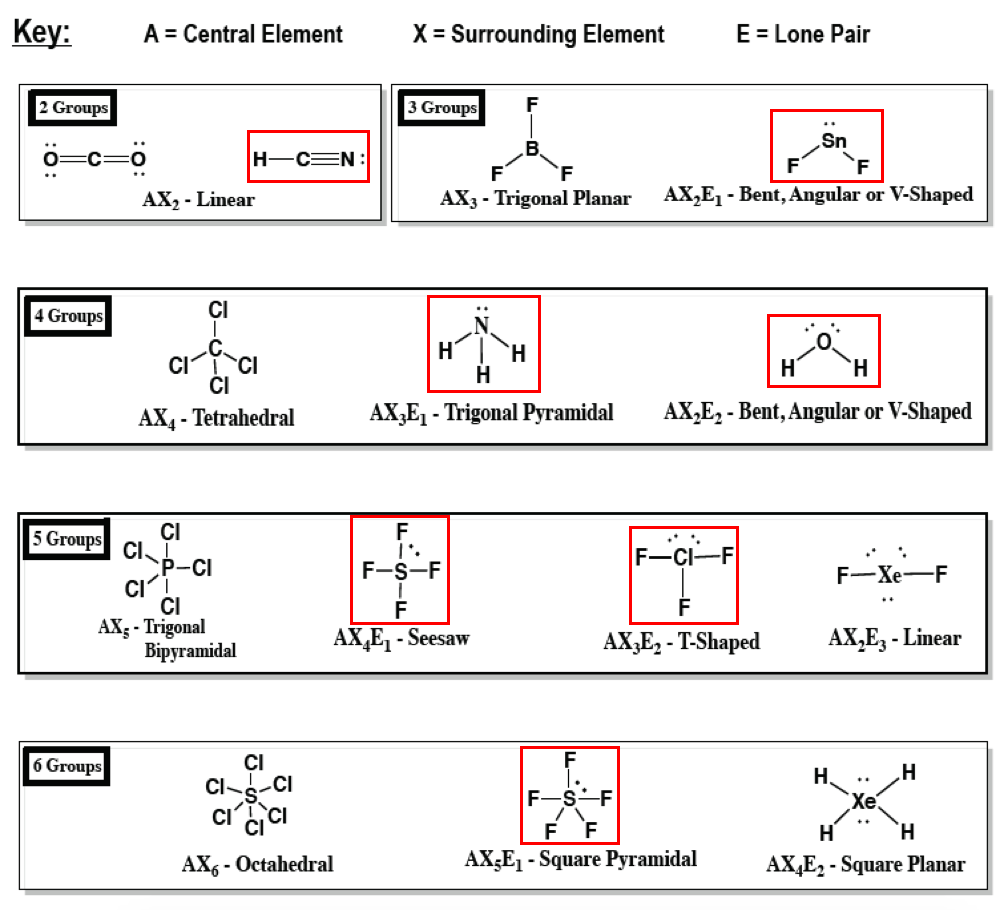
Predict Whether Each Of The Following Mole Clutch Prep

Is Xef4 Polar Or Nonpolar Xenon Tetrafluoride Youtube

Is Xef4 Polar Or Nonpolar Techiescientist

Which Of The Following Have Permanent Dipole Moment Which Of The
Which Element Between If5 Xef4 Sf6 And Ch4 Dipole Moment Quora
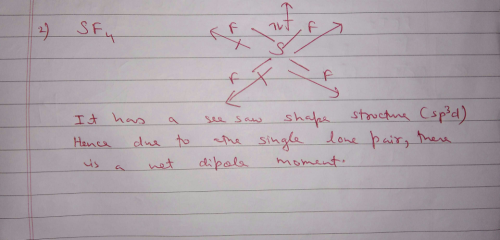
Which Of The Following Would Have A Permanent Dipole Moment 1 Sif4 2 Sf4 3 Xef4 4 Bf3 Moreover Can You Explain The Theory Of Permanent Dipole Moment Edurev Neet Question

Is Xef4 Polar Or Non Polar Xenon Tetrafluoride Youtube
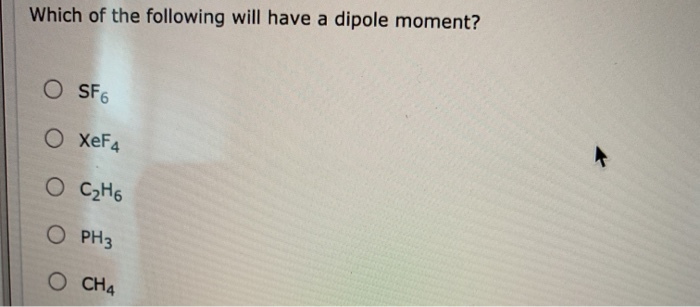
Which Of The Following Will Have A Dipole Moment O Chegg Com
.bmp)
Why Is Xef4 Not A Polar Molecule Chemistry 7346169 Meritnation Com

Why Are The Compounds Co2 Xef2 And Xef4 Non Polar Quora

Which Of The Following Fluorides Of Xe Has Zero Dipole Moment Youtube

Solution Is Xef4 Polar Or Nonpolar Expl Chemistry
Why Are The Compounds Co2 Xef2 And Xef4 Non Polar Quora
F6 Has A Zero Dipole Moment While Sf4 Has A Non Zero Dipole Moment Why Quora

Is Cf4 Polar Or Non Polar Carbon Tetrafluoride Youtube

Is Xef4 Polar Or Nonpolar Techiescientist

Dipole Moment How To Find Molecule Is Polar Or Non Polar Chemical Bonding Class 11th Youtube

Which Of The Following Would Have A Permanent Dipole Moment