Hclo3 Lewis Structure
When we have an H or H2 in front of a polyatomic molecule like CO 3 SO 4 NO 2 etc we know that its an acid. Lewis Dot of the Formate Ion methanoate HCOO-Back.
Draw The Lewis Structure For Hclo 3 From The Skeletal Chegg Com
Chloric acid is a colorless liquid.
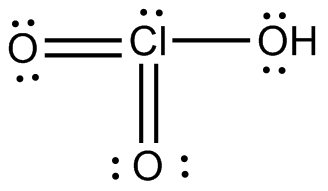
Hclo3 lewis structure. The structure on the right is the lewis electron structure or lewis structure for h 2 o. If the valence shells are filled to the usual limit maximum of 8 what is the sum of the absolute values of all the formal charges in the molecule. Cl atom has one lone pair and 5 bond pairs of electrons.
Your blood brings bicarbonate to your lungs and then it is exhaled as carbon dioxide. Is hco3 a gas. When we see an H in front of a polyatomic structure like the CO3- here that means the H will be attached on the outside of one of the Oxygens.
Lewis Structure of HClO3. There are two C l O double bonds and one C l O single bond. For HCO3- we have a total of 24 valence electrons.
A weak unstable acid it is the active form of chlorine in water. So the structure in which one O atom is bonded to the H atom and Cl has a lone pair the Lewis structure pictured is the structure. The structure of the given compound is pyramidal due to the presence of the lone pair on the chlorine atom.
8cdot22cdot1 18 Total sharedbonding electrons. Draw the Lewis structure for HClO_3 from the skeletal template prated below. The given molecule is HClO3 H C l O 3 Lewis dot structure of HClO3 H C l O 3 is shown below.
Remember Hydrogen always goes on the outside. The number of valance electrons in Cl-atom is seven. It is a member of reactive oxygen species and a chlorine oxoacid.
7 6 1 14 Total electrons needed for octetsdoublets. Determination of the degree of electrolytic dissociation of perchloric acid by vapor pressure Surface and Subsurface Oxygen on Platinum in a Perchloric Acid Solution. The structure of H C l O 3 chloric Acid is shown below.
H atom is attached to O atom via a sigma bond. So well put Carbon at the center and then well put an OH over here. Chloric Acid HClO3 is an oxoacid of chlorine which is a strong acid as its conjugate base is highly stable because the negative charge formed after losing the proton gets resonancely stabilized due to the presence of three oxygen atoms.
It has a role as a human metabolite an EC 3117 acetylcholinesterase inhibitor and an EC 25118 glutathione transferase inhibitor. In the CO32 Lewis structure carbon is the least electronnegative element. Bicarbonate also known as HCO3 is a byproduct of your bodys metabolism.
To my understanding if the H atom is bonded to the central Cl atom the Cl atom would have a formal charge of 1 while the O atom would have a formal charge of -1 if it had another lone pair instead of bonding with the H atom. The exception of course being the hydrogens. Therefore shape of ion is trigonal pyramidal.
The molecule is ax3e and has a triangular pyramidal geometry. Hypochlorous acid is a chlorine oxoacid with formula HOCl. Only one oxygen atom has a -1 charge.
The properly way to determine the Lewis structure based on this example is. Lewis structure of clo3 chlorate anion. It will accelerate the burning of combustible materials and can ignite most on contact.
Therefore overall charge of chlorate ion is -1. Drawing the Lewis Structure for H 2 SO 3. When we have an H or H2 in front of a polyatomic molecule like CO 3 SO 4 NO 2 etc we know that its an acidThis means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
It is used as a reagent in chemical analysis and to make other chemicals. Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. All other atoms do not have charges.
In H 2 SO 3 Lewis structure Sulfur is least electron electronegative atom and goes in the center of the Lewis structure. There are three σ bonds and a one lone pair around chlorine atom in lewis structure of ClO 3-ion. They follow the duet rule 2 electrons.
The lewis structure for hclo3 is as follows. The geometry around Cl is pyramidal. How many charges in atoms of chlorate ion lewis structure.
This test measures the amount of bicarbonate a form of carbon dioxide in your blood. In the HClO 3 Lewis structure Chlorine is least electron electronegative atom and goes in the center of the Lewis structure. 70 More Lewis Dot Structures.
It is corrosive to metals and tissue. Place cl atom as central atom with one pair of valence electrons. Lewis structure for HOCl.
The number of valence electrons in O-atom is six.
Metals Shiny Metallic Appearance Ppt Download

Hclo3 Lewis Structure How To Draw The Lewis Structure For Hclo3 Youtube
Http Www Msubillings Edu Sciencefaculty Handouts Wiles Chem 20116 Solution 20set 201 Pdf
Hclo4 Molecular Geometry Shape And Bond Angles Youtube
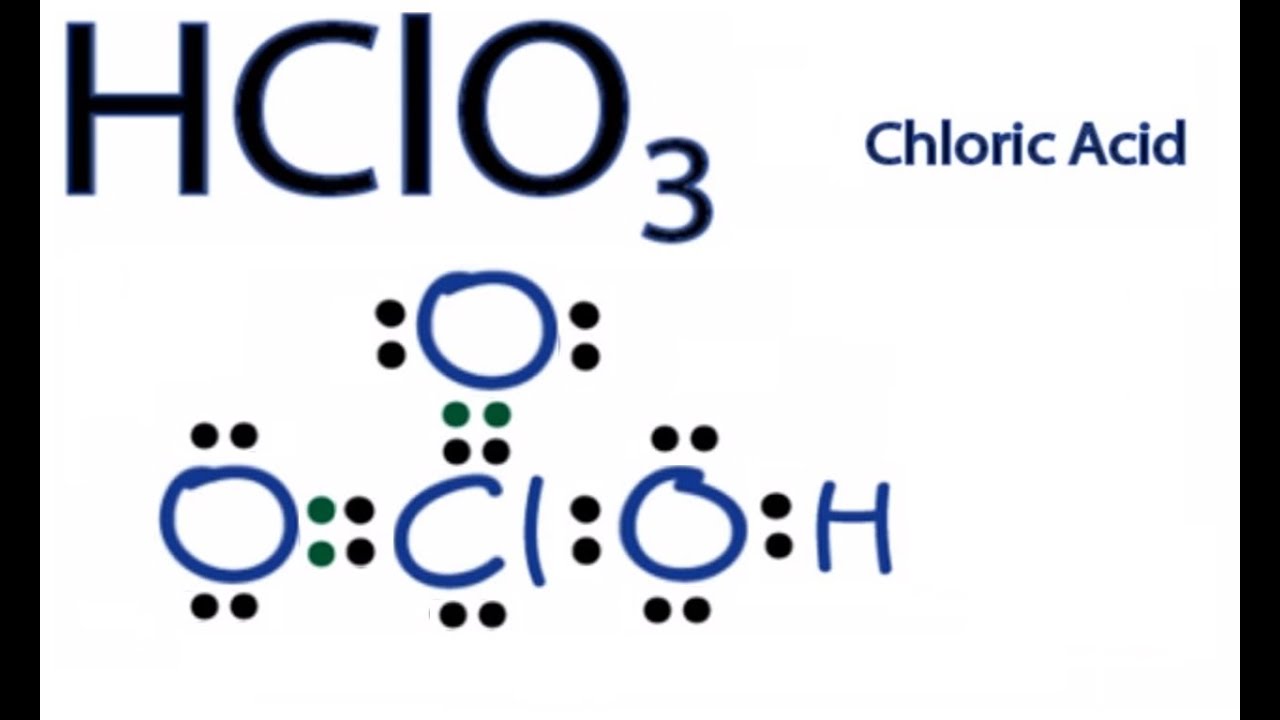
Hclo3 Lewis Structure How To Draw The Lewis Structure For Hclo3 Youtube

Hclo3 Molecular Geometry Shape And Bond Angles Youtube

Hclo4 Lewis Structure How To Draw The Lewis Structure For Hclo4 Youtube

How To Calculate The Formal Charges For Hclo3 Choric Acid Youtube
Why Is Hclo3 A Stronger Acid Than Hclo Quora
Chloric Acid Lewis Structure Chemistry Community

Chemistry For Update Lewis Electron Dot Structures Simple Procedure For Writing Lewis Structures Of Chloric Acid Hclo3

Why Is Hclo3 A Stronger Acid Than Hclo2 Quora

How To Draw The Lewis Structure For Hclo2 Chlorous Acid Youtube

Quimica Estructura Lewis Del Hclo3 Youtube

Which Of The Following Is The Stronger Bro Clutch Prep
Hclo3 Molecular Geometry Shape And Bond Angles دیدئو Dideo

Lewis Dot Strutures Of Hclo3 Brainly In

