Ni3 Lewis Structure Shape
Ni3 lewis structure. I also go over hybridization shape and bond angles.
The shape of a SiO2 molecule.

Ni3 lewis structure shape. Terms in this set 14 the shape of an H2 molecule. State the steric number for the molecule and the name of the molecular geometry. Welcome to 3D Structure.
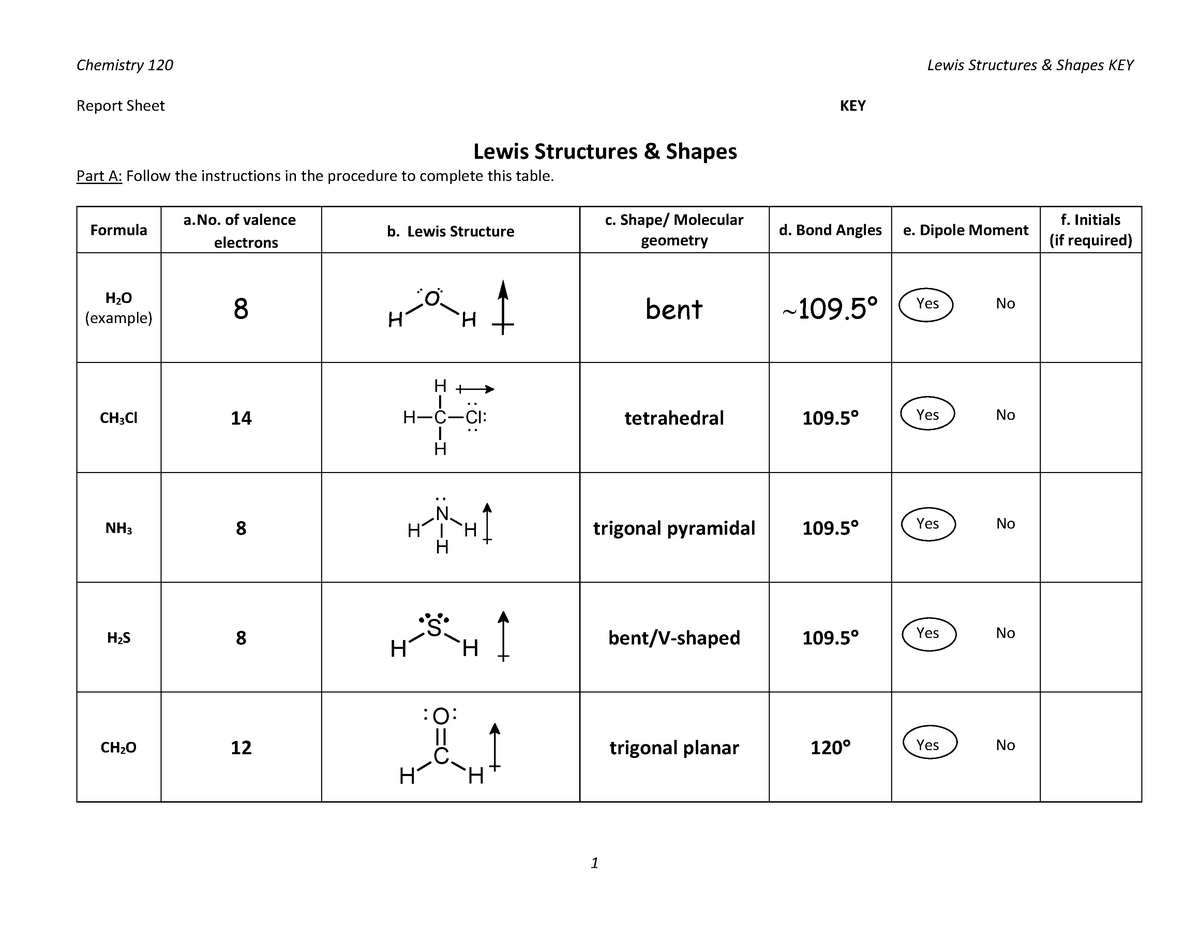
N 2 O i. The chemical formula NI 3 is named nitrogen triiodide. Then identify the LPLP LPBP or BPBP interactions and predict deviations in bond angles.
CCl 2 F 2 d. It is still a polar molecule but has been found to be insoluble in water. Instead the top of the pyramid is occupied by the lone pair and VSEPR.
The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons.
But the molecule has three bonding pairs and one lone pair a VSEPR notation of AX 3 E so its molecular geometry is not the same as the electron-pair geometry. The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair. Its a covalent molecule which means the atoms in the molecule share electrons.
Bond angle of an H2 molecule. Ni3 lewis structure. Note that we do not actually need to draw all the resonance structures to determine the shape as we can see from any one of the three resonance structures that the carbonate ion AB 3 trigonal planar.
Braw the Lewis dot structure for NI. Determine the electron geometry of NI Select Draw Rings More Erase Reset Drawing tetrahedral linear trigonal planar. The shape of a NI3 molecule.
NI 3 is very similar to NH 3 and NF 3. The shape of a CCl4 molecule. There are 24 valence electrons available for the Lewis structure for NO 3-.
Bond angle of a NI3 molecule. State the steric number for the molecule and the name of the molecular geometry. Determine the electron group arrangement around the central atom that minimizes repulsions.
Name Lewis Structure VSPER shape Polar or Non Polar Strongest intermolecular force Formula Formu 02. There are three single bonds and one lone pair of electrons in NH3 molecule. Its a covalent molecule which means the atoms in the molecule share electrons.
Assign an AX m E n designation. Bond angle of an H2S molecule. Answer to Determine the molecular geometry of NI3.
For the NI 3 Lewis structure there are a total of 26 valence electrons available. Draw the Lewis electron structure of the molecule or polyatomic ion. CH 2 O f.
It is termed a contact explosive since it doesnt take much to set it off. Demonstrations will often use a feather to cause the explosion. The shape of a CCl4 molecule.
Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. A Draw the Lewis structure and then determine the shape of NI3 using VSEPR. The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair.
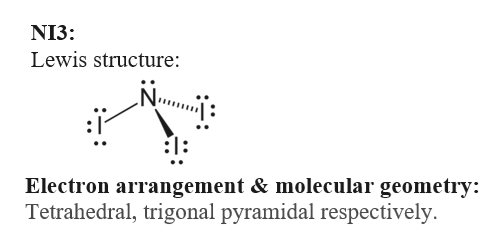
This geometry is the the lowest energy arrangement of the electron groups that minimizes electron pair repulsions Draw the Lewis structure for NI3. AB 4 tetrahedral. Count the total number of electron domains kurrounding the central atom.
AB 3 E 1 trigonal pyramidal. Nitrogen Triiodide and Lewis Structures. The shape of a NI3 molecule.
The shape of a SF6 molecule. Nitrogen triiodide NI3 or I3N CID 61603 - structure chemical names physical and chemical properties classification patents literature biological activities. Does ni3 have a lone pair.
One of these is a non-bonding lone pair. CF 2 H 2 e. Cl 2 r.
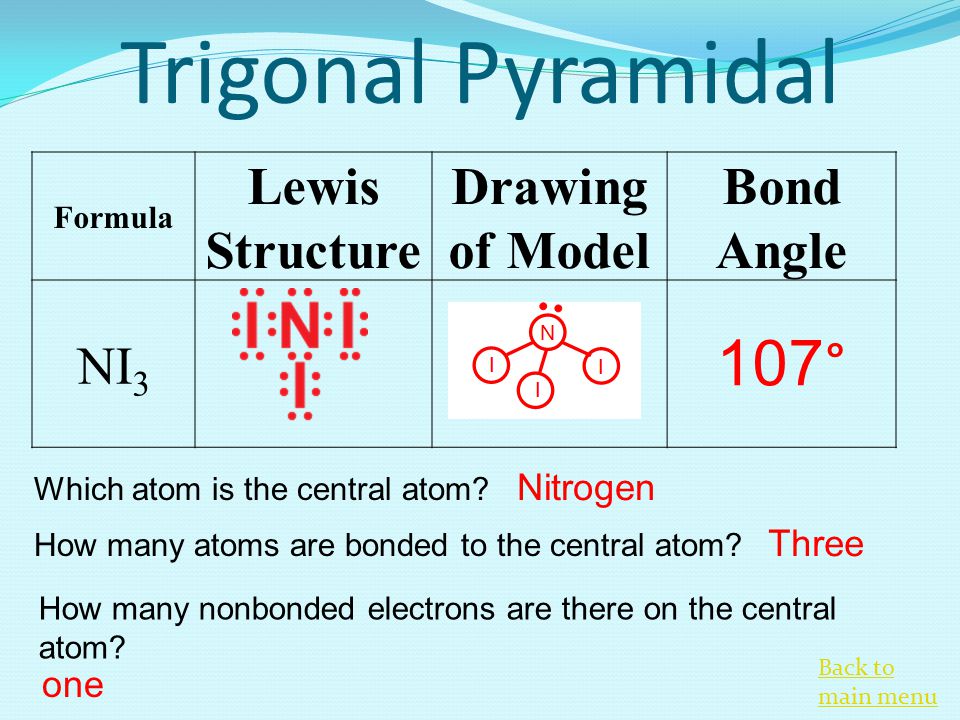
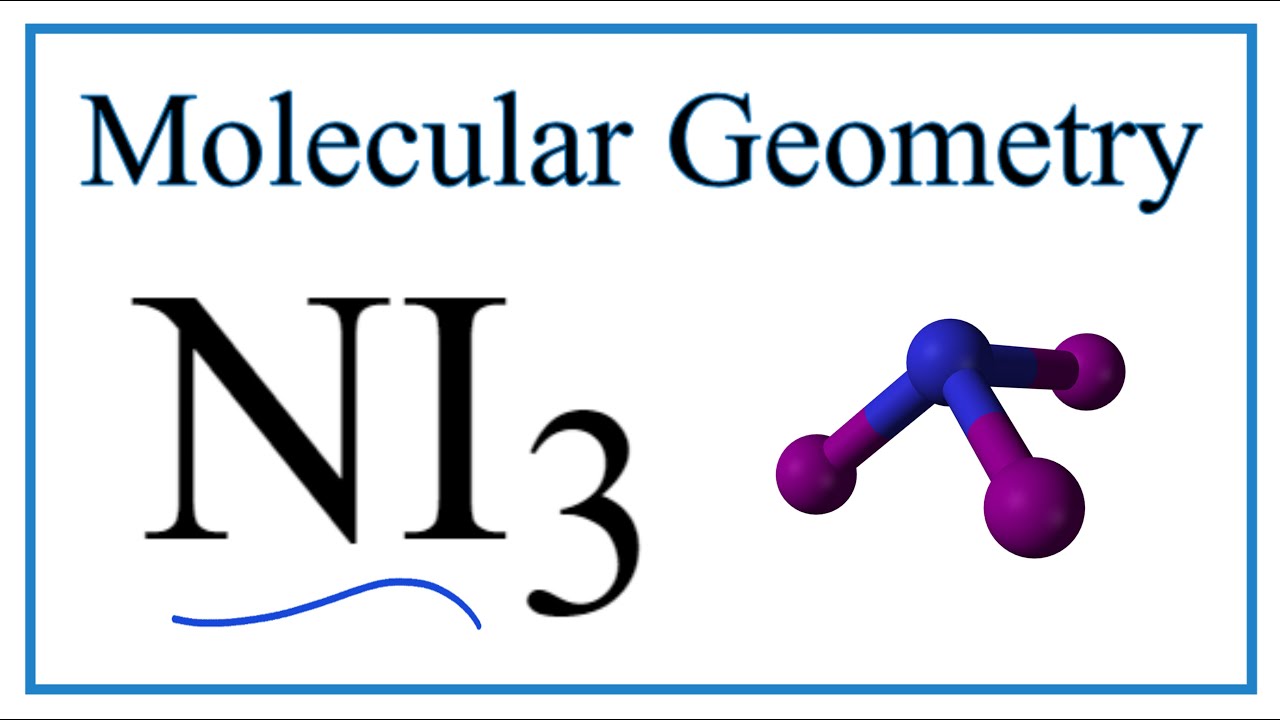
NI3 has a similar structure with NH3. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. H 2 O m.
Be sure to follow octetduet rules for each atom and use the total number of valence electrons available Use your drawing to answer the following questions. Solution for For the following compounds determine. The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry.
A Draw the Lewis structure and then determine the shape of NI3 using VSEPR. NI3 Sketch the proper Lewis structure for this substance.

Ni3 Molecular Geometry Learn Lif Co Id

What Is The Lewis Structure Of Ni3 Study Com

File Nitrogen Iodide 2d Png Wikipedia

How To Draw Lewis Structure Of Ni3 Drawing Easy

The Vsepr Theory By Eunice Yeaineedalife

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Answered Draw The Lewis Structure And Determine Bartleby

Molecular Models Activity Carbon Tetrachloride Ammonia Methane Hydrogen
Covalent Bonding And Nomenclature Ppt Download

Wn Sbi3 Lewis Structure Molecular Geometry Bond Angle Polar Or Nonpolar

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

8 1 Molecular Geometry Properties Of Molecular Compounds Depend Upon 2 Main Things Bonding Molecular Geometry Arrangement Of Atoms In Space Used To Determine Ppt Download
Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube

Lewis Structures Covalent Bonds Hbr Scl2 Mgbr2 Pbr3 Ni3 Bf3
Draw The Lewis Structure For Each Species Below Then Chegg Com